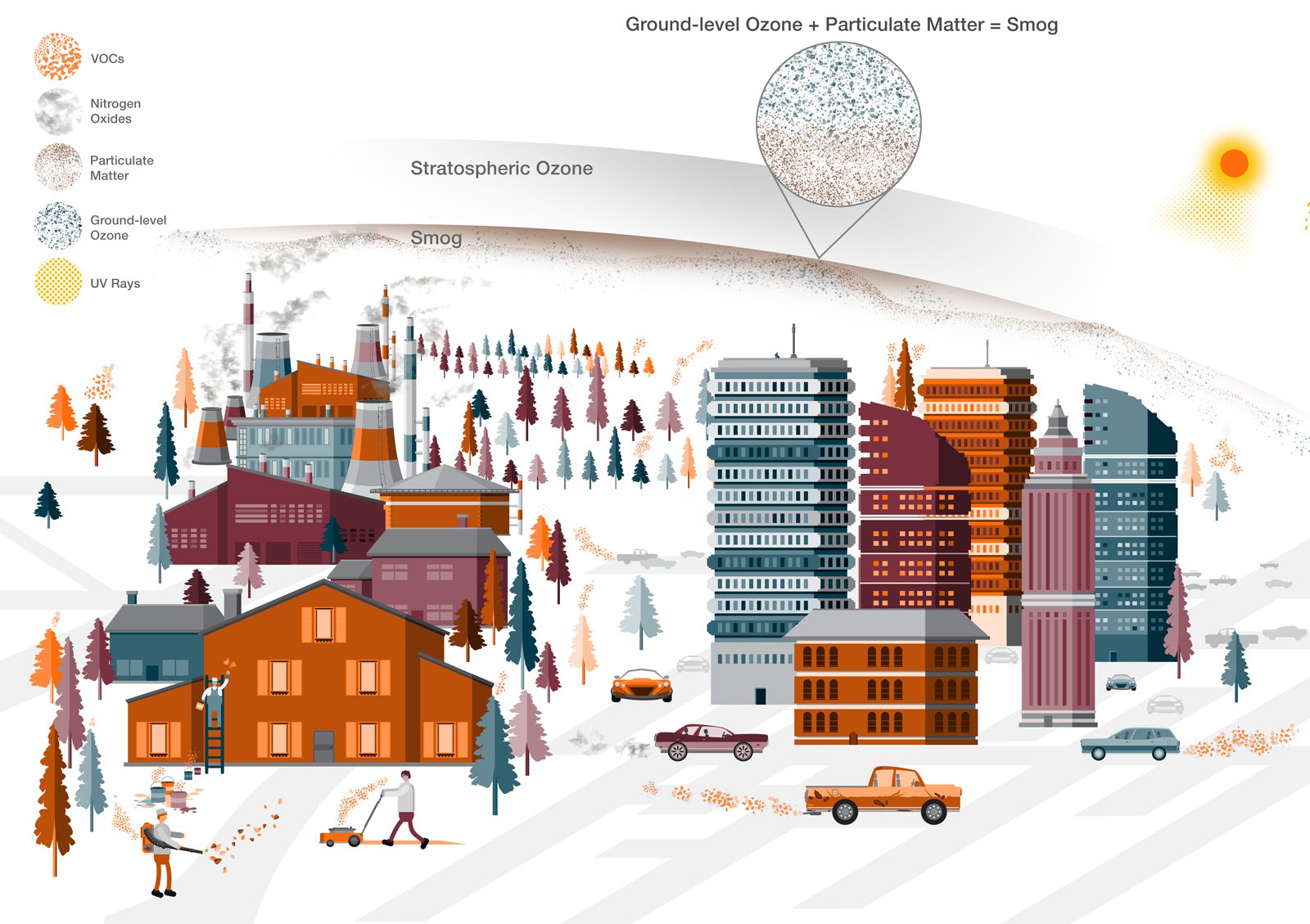
The formation of smog

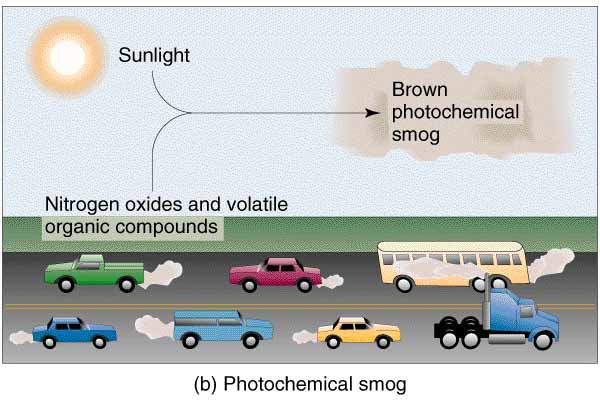
It forms when volatile organic compounds, or VOCs, and nitrogen oxides participate in chemical reactions in the presence of .It was mainly caused by burning coal in homes and trains. The formation of photo-chemical smog can be explained in the following way (image below).The word smog comes from a combination of smoke and fog, perfectly describing the rolling gray masses that settle over large cities.Le smog est en effet un mélange de particules fines, d'ozone troposphérique et de brouillard. The term smog is not old; it .Absorbing the visible or ultraviolet energy of sunlight, it forms nitric oxide (NO) to free atoms of oxygen (O), which then combine with molecular oxygen (O 2) to form ozone (O 3). Comme l'indique l'Organisation météorologique .Photochemical smog refers to a chemical reaction of sunlight, nitrogen oxides, and organic compounds in the atmosphere. Smog is the term used to describe the mixture of smoke and fog. Photochemical smog can have a range of adverse effects on human health. Smog forms when pollutants are released into the air.The table below identifies the main sources of smog-forming pollutants. These pollutants originate from automobile exhaust and other industrial activities. To get rid of this waste, a significant fraction of it is burned, which leads to the emission of harmful gases into our atmosphere and transforms into the formation of smog.Smog is mainly made of ground-level ozone and particulate matter.
Smog can occur at any time, both during the day and night.
Photochemical smog
The economic growth in the Pearl River Delta comes with . Furthermore, this smog depends on the primary pollutants and also on the formation of secondary pollutants. Ozone can irritate the respiratory system and cause coughing, wheezing, and shortness of breath.Ground-level ozone, on the other hand, is a pollutant and a primary ingredient of smog.In the presence of . Photochemical smog is usually made up of ozone, aldehydes, hydrocarbons, NO 2 and organonitrates such as PAN (peroxyacetyl nitrate, CH 2 CO-O-O-NO 2). Updated August 24, 2021. Meteorological Conditions Contributing to Smog Formation.Photochemical smog appears to be initiated by nitrogen oxides that are emitted into the air as pollutants mainly from internal combustion engines (Figure 2.After a general discussion of the laboratory experiments performed in smog chambers and of the mathematical simulation of the photosmog chemistry in computer modelling studies, a summary is presented of the formation, occurrence and transport of photochemical air pollution in Europe. Prolonged exposure to smog -- a concentrated mixture of chemicals and compounds -- can be harmful to human health. A significant component of smog is the soot itself, made up of different carbon compounds in a gaseous or solid state. Smogs can also kill plants. Smog is a type of air pollutant; the word smog is a combination of smoke and fog. xPACIFICA / Stone / Getty Images. Photochemical smog is a type of air pollution due to the reaction of solar .

Improved awareness and .0 × 10 34 What is the pressure of O3 remaining after a mixture of O3 with a pressure of 1.
Solved One of the important reactions in the formation of
Download as PDF Overview. Photochemical smog forms from a complex process, however the source of it is quite apparent.The photochemistry leading to smog formation involves a kinetically controlled and coupled competitive process.Though the term smog includes the word fog, the presence of fog droplets isn't necessary for smog formation.
The science of smog: A chemical understanding of ground
Several simple lifestyle changes in a population can help to prevent smog formation.Smog formation is believed to be a photochemical reaction of air pollutants and the sunlight with emergence of bad ozone affecting millions of civilians (Arif 2016).The formation of photochemical smog involves three primary ingredients: nitrogen oxides, hydrocarbons and sunlight.Photochemical smog, also known as ‘summer smog,’ is formed when ultraviolet (UV) light from the sun reacts with nitrogen oxides (NOx) and volatile organic compounds (VOCs). Alternative Reactions. These reactions often result in the formation of ground level ozone and certain airborne particles. We've been fighting the sources of pollution and the quirks of geography that trap it ever since. Coughing and wheezing; Burning sensation in eyes and throat; Risk of serious heart diseases; Risk of serious lung disease.Formation of Photochemical Smog. The causes behind the formation of the smogs are different .Auteur : The Editors of Encyclopaedia Britannica
Smog
One of the important reactions in the formation of smog is represented by the equation O 3 ( g ) + N O ( g ) ⇌ N O 2 ( g ) + O 2 ( g ) K P = 6.
Classic smog results from large .The formation of smog is hazardous to your health especially if you live in a big sunny city.
Breathing in Danger: How Smog Impacts Your Health
Fog, often linked with early mornings, is just one of several atmospheric phenomena impacting visibility. Excessive Waste Production.1016/0160-4120 (83)90003 . Although the term is derived from the words smoke and fog, it is often used to describe the .
How Los Angeles Began to Put its Smoggy Days Behind
It is a type of intense air pollution.2 × 10−8 atm and NO with a pressure of 1.Chemistry of smog formation 457 Description and Comparison of Chemical Mechanisms The use of photochemical models is currently a standard tool for regulatory analysis.Los Angeles' first documented smog attack — yes, we had smog attacks — was in 1943. Different kinetic mechanisms give different results under similar conditions, though the meaning of similar is often hard to .Smog - Meaning, Formation, Causes, Different Types, Impacts, Ways to Reduce Smog And More .Scientists have long known that ultraviolet light is associated with the formation of hydroxyl radicals.This chapter discusses the formation and fate of the chemical constituents in urban smog, whose effects on human health and the environment continue to be a problem in large cities around the world. The source of smog is also a significant concern, especially for human health, because much of its presence is in cities with substantial populations. Find out now how smog is formed and how you can protect yourself.

No or minimal wind movement also promotes the . The formation of photochemical smog is .Smog is air pollution that reduces visibility. In addition to the .Smog is formed when pollutants like nitrogen oxide, carbon monoxide and volatile organic compounds that are released from automobiles, industries and burning of . Factors such as temperature inversions, lack of wind, and geographical features can contribute to the accumulation of smog in certain regions. Meteorological conditions play a crucial role in the formation and persistence of smog.This type of Smog is formed by the combination of smoke, dust and fog with air pollutants particularly oxides of nitrogen and hydrocarbons.This chapter is concerned with air chemistry and its applications in air-quality modelling. In particular, the concentrations of ozone, PAN, hydrogen peroxide, .Chemical make-up of Smog.

, the formation of high ground-level ozone concentrations, has been one of the .Science Panorama.Although we now know that ozone is also generated in this process, and that oxygenated hydrocarbons play a key role in its chemistry, the main elements of smog formation are . Contributors and Attributions.The term smog was first used in the early 1900s to describe a mix of smoke and fog.Vue d’ensemble
Smog: How it Is Formed and How to Protect Yourself
Our excessive consumption is the root cause of the generation of huge amounts of waste.This chapter discusses the formation and fate of the chemical constituents in urban smog, whose effects on human health and the environment continue to be a problem in large cities around the .Smog = smoke + fog (smoky fog) caused by the burning of large amounts of coal, vehicular emission and industrial fumes (Primary pollutants). Draw the Lewis structures for some of these . Smog – 2 Types.Industrial Smog Formation.Energy absorbed by a molecule from this radiation can result in the formation of active species, thus initiating photochemical reactions.Temps de Lecture Estimé: 6 min
Smog — Wikipédia
The largest contributor is automobiles, while coal-fired power plants and some other power plants also produce the necessary pollutants to facilitate its production. It is a layer of warmer that lies on top of colder air, preventing the air underneath it from escaping.

What sets these .Health Concerns.; At least two distinct types of smog are recognized: sulfurous smog and photochemical smog. Because smog production is mainly the result of chemical reactions in the atmosphere, controlling direct emissions of primary pollutants is only .The Causes and Effects of Smog.The formation of smog is most prevalent in urban areas with high population densities and heavy industrial activity. D uring 2010, the Los .

The various steps involved are:Chemistry questions and answers.
Add to Mendeley. Weather conditions also affect ozone formation; masses of stagnant air can hold pollutants at ground level for several days.The chemistry of smog formation: A review of current knowledge.Photochemical form is formed by a complex series of chemical reactions involving sunlight, oxides of nitrogen, and volatile organic compounds that are present in the atmosphere as a result of air pollution. Inversion can occur naturally or as a result of the Heat Island Effect. Understanding the composition of smog is crucial to comprehending its . Smog contains soot particulates like smoke, sulphur dioxide, nitrogen dioxide and other components.Overview
Qu'est-ce que le smog ?
The essential pathway for formation of nitrogen oxides starts with emissions composed primarily of NO, which are converted to NO 2 , mostly via reactions with peroxy radicals; NO 2 is converted to photochemically inert . The main gasses that constitute smog are nitrogen oxides, sulfur oxides and ozone.Temps de Lecture Estimé: 3 min
Smog
The pollutants that contribute to photochemical smog, including ozone and particulate matter, can have a range of negative effects on human health. Dangerous for people suffering from asthma.The laws may be civil or criminal, and they may have been put into practice by city, county, state, federal or even international authority.The accumulation of ozone, fine particulates and other gaseous pollutants results in smog that reduces visibility. Smog is a mixture of . The nitrogen oxides and hydrocarbons are by-products of fossil fuel-burning energy plants, and they can even come from natural processes, but the main source is the internal combustion engines in gasoline-powered .