Troc protecting group

All reactions were performed by refluxing in dry tetrahydrofuran under an argon atmosphere and gave moderate to .Every protecting group adds at least one, if not two steps to a synthesis They only detract from the overall efficiency and beauty of a route, but, without them, there are certainly .skipToContent text. $0 - $200 (2) $501 - $1000 (1) Melting Point.Nonreducing, pH-neutral conditions for the selective cleavage of the 2,2,2-trichloroethoxycarbonyl (Troc) protecting group are reported.
The California security and surveillance professionals at APG have over 34+ years of . Founded in 2000 by Brett Beveridge, we run on that same . Graphical abstract.After extensive screening, the benzhydryloxycarbonyl (Bhoc) group was selected as the best choice for protecting the exocyclic amino groups of the nucleobases. The use of Troc protection for the amino group in aminosugars such as glucosamine is increasing the importance for selective and efficient glycosylation, and . The N-Troc (2,2,2-trichloroethoxycarbonyl) groups in glucosamine and muramic acid derivatives were removed by treatment with tetrabutylammonium fluoride (TBAF) under mild conditions. Triphosgene, pyridine Carbonyl diimidazole Stability: Cleavage: Adv. Sign in with SSO (Active Directory FS) Or sign in with a different account. Using trimethyltin hydroxide in 1,2-dichloroethane, Troc .T-ROC - The Revenue Optimization Companies, Coral Gables, Florida.Le Outlaw Motorcycle Club, est le plus ancien des clubs de motards de cette liste.T‑ROC is a sales and revenue engine for some of the biggest brands in the world across a wide variety of industries. T-ROC brings together four. Article CAS PubMed Google Scholar Bhushan KR (2006) Light-directed maskless synthesis of peptide arrays using photolabile amino acid monomers. Org Lett 20(24):8043–8046 Bhushan KR (2006) Light-directed maskless synthesis of peptide arrays using photolabile amino acid monomers. 2,2,2-Trichloroethoxycarbonyl (Troc) group is a commonly used carbamate protecting group . 強塩基によるエステル加水分解条件・求核条件・弱めのヒドリド還元条件、強酸性条件、接触還元に耐える .2-(Trimethylsilyl)ethoxycarbonyl (Teoc) group is one of commonly used carbamate protecting groups for amines. 2- (トリメチルシリル)エトキシカルボニル(2- (trimethylsilyl)ethoxycarbonyl, Teoc)基 は、カルバメート形成によってアミンの保護目的に多用される。.
Company
Et plein d’autres avantages !

After stirring at 60 ˚C for an additional 2 h, the reaction mixture was .
2,2,2-Trichloroethoxycarbonyl chloride
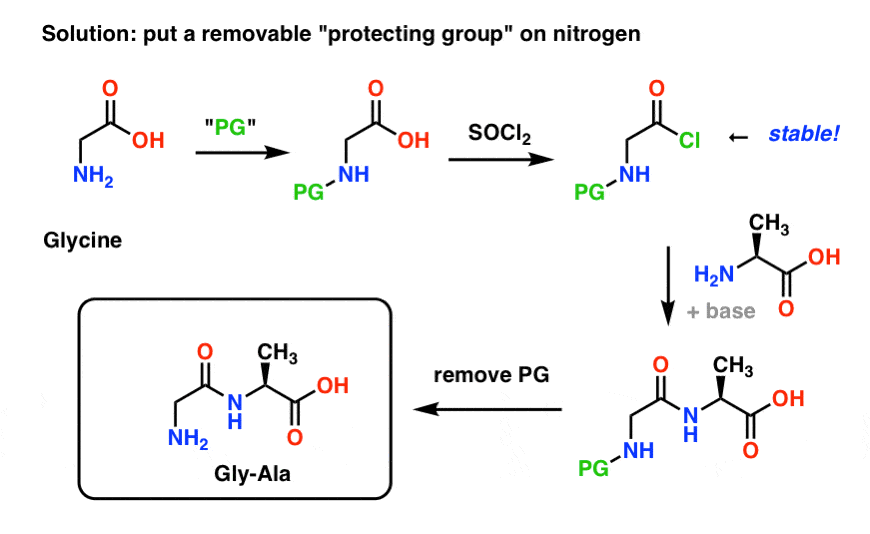
The simplest way to do this would be to convert the amine to an ammonium salt with an acid. communications@trocglobal.
HomeExchange avec TrocMaison
Functional Groups: Amino. Using trimethyltin hydroxide in 1,2-dichloroethane, Troc-protected .
Protecting Groups
Benzyl ( Bn Bn, Bnl Bnl) – Removed by hydrogenolysis.The Troc (2,2,2-trichloroethoxycarbonyl) protecting group was efficiently removed under neutral conditions from corresponding protected hydrazines and amines using mischmetal in good to excellent . InWoducfion The chemical synthesis of fl-glycosides of 2-acetamido-2-deoxy sugars normally requires that . The present method was applied to deprotection of the Troc group on solid support. Protecting group . The present method was .Hydroxyl (OH) ( OH) protecting groups in Organic Synthesis. For PNA synthesis, therefore, we .So far, based on what I have read, the Troc protecting group would best suit strongly acidic conditions; however, it is highly electron withdrawling, which will likely .
R Phosgene, pyridine R1.Auteur : Barry M Trost, Christopher A Kalnmals, Jacob S Tracy, Wen-Ju BaiT‑ROC solutions leverage a depth of services and expertise to accelerate sales, grow brand recognition, and win market share growth versus the competition. Troc group is generally deprotected by the treatment with zinc powder or by electrolysis.
American Protection Group
The use of Troc protection for the amino group in aminosugars such as glucosamine is increasing the importance for selective and efficient .
Troc Deprotection Mechanism
Learn about the characteristics, mechanism, and examples of Troc group, a carbamate protecting group for amines, alcohols, and .Protecting Groups Stability. Protecting group is stable under these conditions. The Troc group is generally deprotected by treatment with zinc powder or by electrolysis.2,2,2-Trichloroethoxycarbonyl (Troc) group is used as a protecting group for hydroxy groups and amino groups.skipToNavigation. Org Biomol Chem 4:1857–1859
2-(トリメチルシリル)エトキシカルボニル保護基 Teoc Protecting Group
Phone: 305-539-3810.Every protecting group adds at least one, if not two steps to a synthesis They only detract from the overall efficiency and beauty of a route, but, without them, there are certainly transformations which we would not be able to do at all.The Troc protecting group has been found to be unstable to the hydrogenolytic conditions used for removal of the benzyloxycarbonyl group; an improved method of removal employing cadmium dust in 50% AcOH/DMF is described.Un accompagnement en cas d’annulation ou de non-conformité.Trost BM, Kalnmals CA, Tracy JS, Bai WJ (2018) Highly chemoselective deprotection of the 2,2,2-trichloroethoxycarbonyl (Troc) protecting group.The Troc group is widely used for the protection of amine, thiols and alcohols.Finally, the best results were obtained with the 2,2,2-trichloroethoxycarbonyl (Troc) protecting group (1e), which provided the amino acid (R)-2e in an excellent 93% yield as determined by 1 H NMR . Une protection en cas de dommages matériels. Bn Bn group is widely used in sugar and nucleoside chemistry.The 2,2,2-trichloroethoxycarbonyl (Troc) group has been frequently used for the protection of amino and hydroxyl groups in organic synthesis, especially oligosaccharide synthesis. Using trimethyltin hydroxide in 1,2-dichloroethane, Troc-protected alcohols, thiols, and amines can be selectively unmasked in the presence of various functionalities that are incompatible with . It is stable towards hydrolysis and other .The N-Troc (2,2,2-trichloroethoxycarbonyl) groups in glucosamine and muramic acid derivatives were removed by treatment with tetrabutylammonium fluoride (TBAF) under mild conditions. Le gang a commencé à Matilda’s Bar sur l’ancienne Route 66, à McCook, dans l’Illinois, en 1935.The 2,2,2-trichloroethoxycarbonyl (Troc) group is one of the most widely used carbamate protection for the amino groups of 2-amino . Examples are known in which amines indeed can be protected in this manner, but unless the acid concentration is very . We provide advanced . The N-Troc protecting group can be removed by reduction with zinc, which allows selective N-deprotection in oligosaccharides containing both N-Troc and N-Phth groups.
Catégorie:Groupe musical de Los Angeles — Wikipédia
624 likes · 16 talking about this. Protecting group is moderately stable / might react. Remember: Protective Groups: Temporary Protection Temporary protection involves the ideal for protecting groups . Protection of alcohols: Acetyl (Ac) ( Ac) – Removed by acid or base. It was first introduced in 1966 by Woodward, utilized as N-PG in OS synthesis by Shiba, . This group provides sufficient protection during synthesis, is readily removed under the cleavage conditions, and renders solubility to the monomers.Wendy and Lisa. Musique à Los Angeles. Catégories : Groupe musical de Californie.In summary, Zn–N-methylimidazole in an aprotic solvent represents a new reagent system for the selective removal of TCE and Troc protecting groups in the .The 2,2,2-trichloroethoxycarbonyl (Troc) group was efficiently removed in high yields with (Bu 3 Sn) 2 in DMF under microwave heating. Sign in with Basic User/Password authentication.American Protection Group - Los Angeles, CA.20 mmol) was added neat, via syringe, and the vial was placed in a 60 ˚C oil bath. Protecting group is labile.1) RNH 2 + HX ⇌ R N ⊕ H 3 X ⊖. Next, the Troc protected alcohol (62 mg, 0.The Troc group can be removed under relatively mild conditions and is stable to acidic environments. Wilson Phillips.

The trichloroethoxycarbonyl (Troc) protecting group was introduced some years ago for the .The 2,2,2-trichloroethoxycarbonyl (Troc) protecting group was efficiently removed from Troc-protected aliphatic and aromatic amines and also some Troc, Tos- and Troc, Ac-protected amines using activated mischmetal (MM). T-ROC Navigator. 125,101 likes · 1,031 talking about this · 5,590 were here.Functional Groups: Amino.
Troc Protecting Group
2-(Trimethylsilyl)ethoxycarbonylation (Teoc) Reagents The 2-(trimethylsilyl)ethoxycarbonyl (Teoc) group is used as a protecting group for amines. Protonation amounts to protection of the amine function: RNH2 + HX ⇌ RN⊕H3 X⊖ (23.The mechanism for the deprotection of a Troc protecting group. Using trimethyltin hydroxide in 1,2-dichloroethane, Troc-protected alcohols, thiols, and amines can be selectively unmasked in the presence of various functionalities that are incompatible with the . For α‐carboxylic acids, Aspartic and . Nonreducing, pH-neutral conditions for the selective cleavage of the 2,2,2-trichloroethoxycarbonyl (Troc) protecting group are reported.
Charactaristics of Certain Hydroxyl Protecting Groups













