Unit cell calculations

Table 1 shows such a calculation for the unit cell of sodium chloride.To use this online calculator for Density of Unit Cell, enter Number of Atoms (z), Mass of Atom (M) & Edge Length (a) and hit the calculate button. The structures of the two unit cells are shown in Fig.

Mass of the unit cell = no.25 × 10 − 24 g ) crystallises . - the mass density of CsCl.Regarder la vidéo2:23Calculations involving unit cell dimensions are dealt here in this video.To determine this, we take the equation from the aforementioned Simple Cubic unit cell and add to the parenthesized six faces of the unit cell multiplied by one-half (due to the .
Cite this chapter. BCC’s largest interstitial size is tetrahedral, which is 29% of the atom size. Put your understanding of this concept to test by answering a few MCQs.In this section, we investigate a larger unit cell that includes 16 atoms. Crystalline solids have regular ordered arrays of components held together by uniform intermolecular forces, whereas the . The latter however can only be true if the side-walls are excluded, since a numerical farfield integration has to be performed on the unit cell boundaries otherwise.The developed framework prescribes all stress ratios in unit cell calculations. Please note that for RCS calculations with the Frequency . In a simple cubic lattice, the unit cell that repeats in all directions is a cube defined by the centers of eight atoms, as shown in Figure 10.For unit cells generally, . It highlights the key differences between the simple cubic unit . All crystal lattices are built of repeating unit cells. You will also learn how to generate . In “Surface Wave Bloch Mode Synthesis (SW-BMS) Method”, the numerical calculation process of the main procedures of the SW-BMS method is given, which includes domain .
Unit cell
Simple tetragonal has 1 atom per unit cell, lattice constant a = 2R (ac), Coordination Number CN = 6 (4 or 2), .Temps de Lecture Estimé: 9 min
Unit Cell
The density can be expressed as: ρ= (z×M)/ (a3×NA) ∴ρ=1×M/a3×NA. Meanwhile, Tekoglu . Number of ions Na+ Cl– at 8 corners - 8 × ⅛ = 1 on 12 edges 12 × . Here is how the Volume of Unit cell calculation can be explained with given input values .How to calculate Volume of Unit cell using this online calculator? To use this online calculator for Volume of Unit cell, enter Edge Length (a) and hit the calculate button.The packing fractions of the crystal structures shown above can be calculated by dividing the volume of the atoms in the cell by the volume of the cell itself.
Dilution Calculator
If you are talking about the HCP crystal structure, the conventional HCP unit cell has 6 atoms, . of atoms in the unit cell × mass of each atom = Z × m. In a body-centered density of unit cell formula, there are two atoms. Cell of the Primitive Unit. The face-centered cubic (fcc) unit cell of aluminium and copper.Calculation of density of unit cell. Also from quantitative aspect of atoms, mass of one atom can be written in terms of Avogadro Number ( N A ) and molar mass of atom ( M), that is, Volume of Unit Cell = a 3.Unit Cell Calculations 3. In this tutorial you will learn how to generate unit cells for various lattice types with Atomsk.

Three dimensions. Test your Knowledge on Density of unit cell! Cells that have integral multiple of lattice constant (a) are multiples of .
Simple Monoclinic Unit Cell
For example, the hexagonal conventional cell of Bi 2 Te 3 has 15 atoms with 45 vibrational modes, which is three times larger compared to that of the primitive unit cell. Click ‘Start Quiz’ to begin! Select the correct answer and click on the “Finish” button Check your score and answers at the end of the . ∴Density d = mass of unit cell/volume of unit cell = ZM/a 3. From the calculation we can see that each unit cell contains 4 Na+ ions and 4 Cl– ions and hence the formula of sodium chloride is NaCl and it has no overall charge.Cu is the prototype for FCC. In a primordial density of unit cell formula, there is only one atom. Pure materials never take this crystal structure, and it exists only mathematically.each particle within the unit cell. You might need: Calculator., and Pollmann, J.2, lattice parameter ain a. { How many atoms there are in the unit cell nat=2: two atoms In “Calculation of Band Structures”, the unit cell and Bloch boundary conditions of a periodic structure are listed. Two dimensions. [56] found that material anisotropy significantly affects the void growth of different metal sheets under various stress states. A unit cell is the smallest portion of a crystal lattice that shows the three-dimensional pattern of the entire crystal.Temps de Lecture Estimé: 6 min
Unit Cells
633), coordination number CN = 12, and Atomic Packing Factor APF = 74%.The volume of an monoclinic unit cell is , obtained by multiplying the square area of the base by the height of a cell.
Farfield Calculation Overview
The simple tetragonal unit cell can be imagined as a cube that is slightly taller or shorter in one direction, with an atom on each corner. Mass of an atom present in the unit cell = m/N A. Don’t worry, I’ll explain what .
Muddiest Point
In addition to this tutorial, it is recommended to read the documentation page of the mode --create.The Simple Cubic Lattice
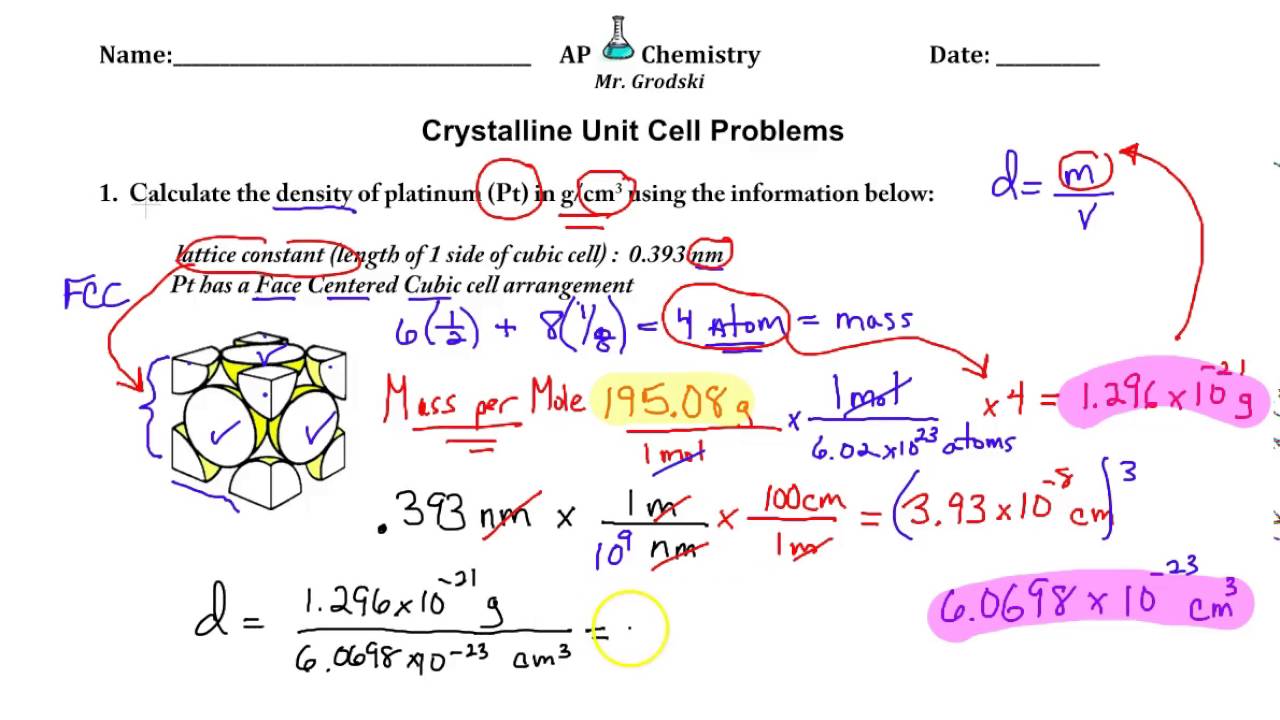
Unit Cell Calculations
Here is how the Density of Unit Cell calculation can be explained with given input values -> 2E-23 = 35*0. m = mass of a single atom.To calculate the density of a solid given its unit cell.
3: An atom in a simple cubic lattice structure contacts six other atoms, so it has a coordination number of six.Since an atom at a corner of a simple cubic unit cell is contained by a total of eight unit cells, only one-eighth of that atom is within a specific unit .The farfield calculation itself then can be deemed more accurate than the numerical farfield integration, and is faster.The face-centered cubic unit cell contains a single octahedral hole within itself, but octahedral holes shared with adjacent cells exist at the centers of each edge.
Unit Cell Calculations
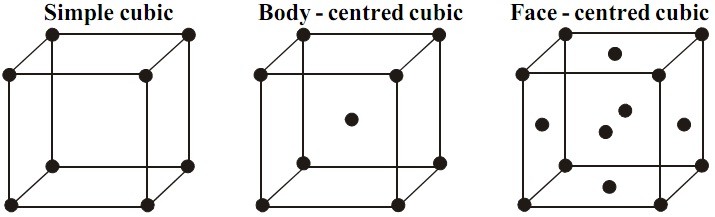
A monocristal of NaCl has a mass of 410 g; Immersed in a beaker filled to the brim with petrol, it causes overflow of 190 cm 3 liquid.The total number of particles in a unit cell can therefore be determined by counting the number of particles in each position of the unit cell (at a corner, edge, face or in the . In a unit cell, an atom's coordination number is the number of atoms it is touching. The unit cell is the parallelepiped built on the vectors, a, b, c, of a crystallographic basis of the direct lattice.Mathematically mass of unit cell is the product of number of atoms “n” and mass of one atom “m” i.The entire structure then consists of this unit cell repeating in three dimensions, as illustrated in Figure 12. Its volume is given by the scalar triple product, . Wigner–Seitz cell.FCC’s largest interstitial site is octahedral, which is 41% of the atom size.1 Fractional Coordinates The location of any point within a unit cell (oblique or orthogonal) by means of three coordinates (x, y, z) and three basis . Many metals, like aluminium, copper, or nickel, crystallize into the fcc lattice. In the first image, a cube with a sphere at each corner is shown. Added to the single hole contained in the middle . A diagram of two images is shown. Calculate (answers at the bottom): - the molar mass of CsCl.Sketch the three Bravais lattices of the cubic system, and calculate the number of atoms contained in each of these unit cells.In the LUC method, a unit cell that is a multiple of either the Bravais or primitive cell of the diamond structure is used to represent the bulk of crystals [14]. A crystal can be thought of as the same unit cell . A 16-atom conventional unit cell provides more degrees of .inAuteur : Scholarswing5^3)* [Avaga-no]). a conventional cell has been chosen on a case-by-case basis by crystallographers based on convenience of calculation. These conventional cells may have additional lattice points located in the middle of the faces or body of the unit cell.Volume of a unit cell = a 3. Placing the required values in .The present paper is organized as follows.
Cubic Cell Calculator
The Face-Centered Cubic (FCC) unit cell can be imagined as a cube with an atom on each corner, and an atom on each face. Each of these twelve edge-located sites is shared with four adjacent cells, and thus contributes (12 × ¼) = 3 atoms to the cell.
Interstitial Sites: Size, Types, Applications, And Calculations
It is one of the most common structures for metals. (Top) Primitive cell.SR has 1 atom per unit cell, lattice constant a = 2r (or ), Coordination Number CN = 6, and Atomic Packing Factor APF ranging from 0. FCC has 4 atoms per unit cell, lattice constant a = 2R√2, Coordination Number CN = 12, and Atomic Packing Factor APF = 74%. Each sphere has a coordination number of 6 and there is 1 atom per unit cell. of atoms present in one unit cell. Download book EPUB. Proportional and non-proportional loadings can be imposed on unit cells. Conventional cell. - the molar volume of CsCl.Atomsk can be used to generate certain types of unit cells, thanks to the mode --create. The simple cubic has a sphere at each corner of a cube. Show how alternative ways of stacking three close . (practice) | Khan Academy. You need to specify: { What is the Bravais lattice ibrav=2, meaning fcc lattice { How many and which parameters are needed to completely de ne Bravais lattice geometry just one: celldm(1)=10.Based on unit cell calculation, Hosseini et al. Element Z (Atomic mass- 125. First Online: 23 January 2021. Google Classroom. Numerical Problems in .This video addresses issues students have with unit cell calculations by doing an activity that involves many related calculations.To view other chapter videos please purchase from our site https://www.A unit cell is the smallest representation of an entire crystal.Some of these LUCs are given in Table 1 in terms of the 8 atom Bravais unit cell or 2 atom primitive diamond cells. See the command options and how to invoke each of them at Choice of force calculator. This video is an update o. The interfaces for VASP, WIEN2k, Quantum ESPRESSO (QE), ABINIT, Elk, SIESTA, CRYSTAL, DFTB+, TURBOMOLE, FHI-AIMS, CASTEP, ABACUS, and LAMMPS are built in to the usual phonopy command.









.jpg)