What are ideal gases
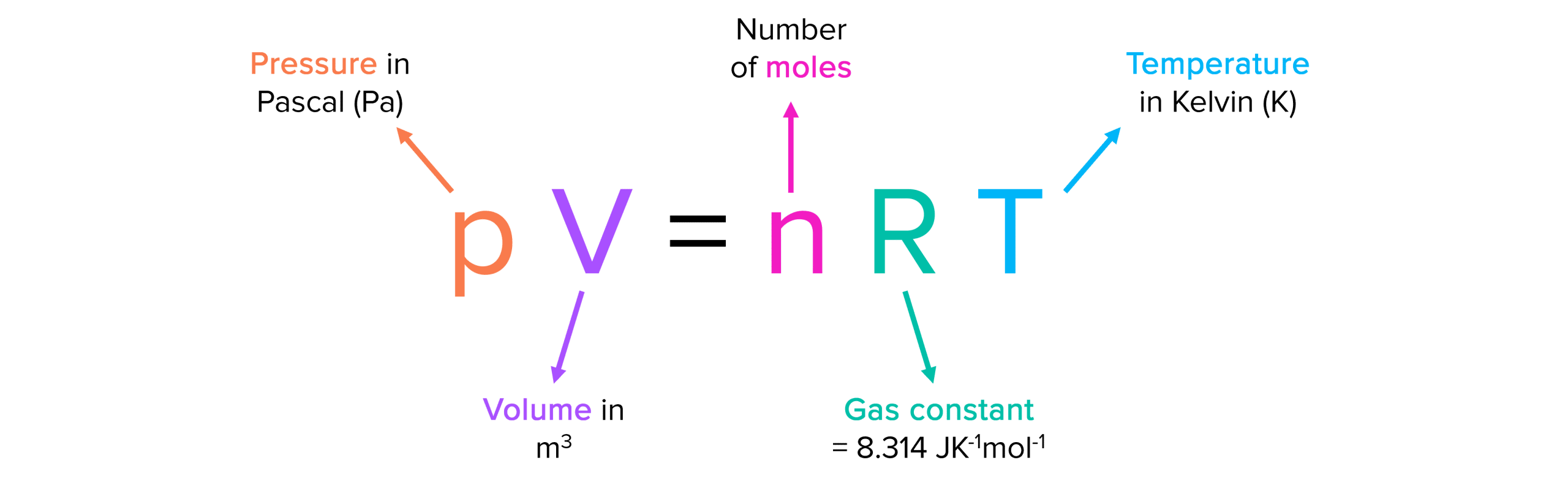
An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this .
Chemistry Study Guide for Gases
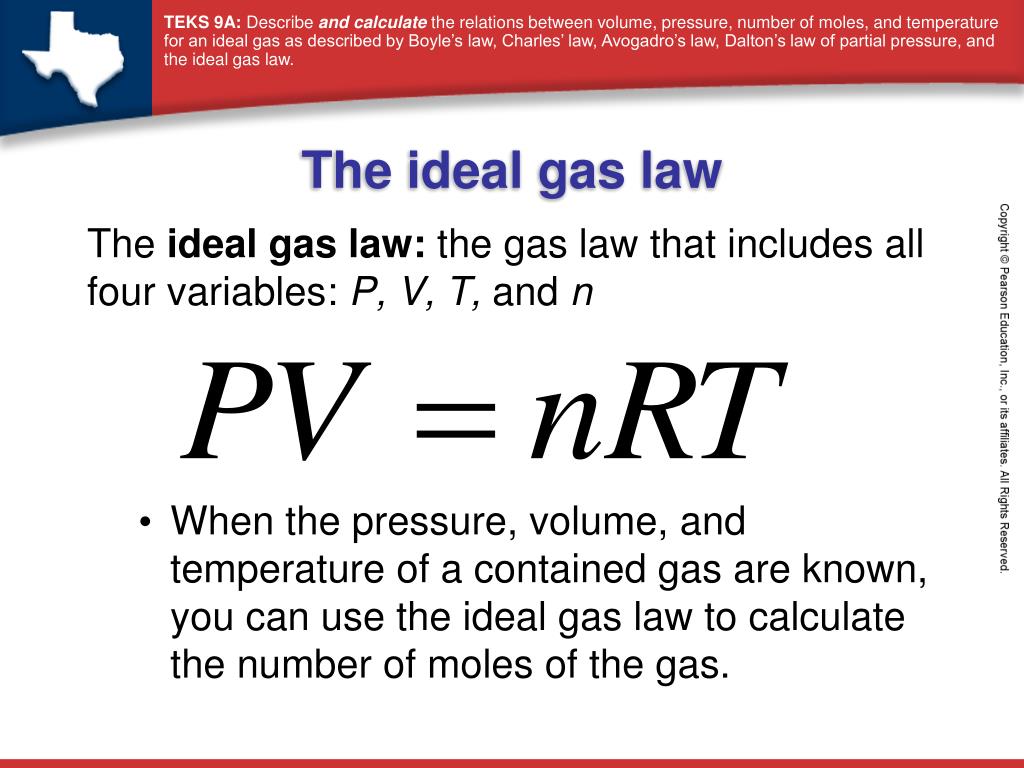
Balises :Equation of Ideal GasGasesIdeal Gas ConstantIdeal Gas Law Variables Standard temperature and pressure (STP) is 0°C and 1 atm.The Ideal Gas Law is a single equation which relates the pressure, volume, temperature, and number of moles of an ideal gas.Using the ideal gas law and the temperature in kelvins (298 K), we calculate the pressure: P = nRT V = 7. 3 Molecules can collide with each other and with the .Perfect gas nomenclature. Calculate the partial pressures of CO 2 and N 2. the basics of the Kinetic Molecular Theory of Gases ( KMT) should be understood.An ideal gas is a theoretical gas composed of many randomly moving point particles that do not interact except when they collide elastically. To do so, the gas needs to completely abide by the kinetic . Deviations from ideal gas law behavior can be described by the van der Waals equation, which .
11 Ideal and Non-Ideal Gases
It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Nevertheless, the reason why. The ideal gas law can be written in terms of .We can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°C and 1 atmosphere pressure.An ideal gas is a gas that obeys the ideal gas law, which relates pressure, volume, and temperature.Advertisements.1 – Ideal Gas in a Cubical Container.An ideal gas is a gas with particular characteristics.Derivation of the Ideal Gas LawGases are a fundamental state of matter. If chlorine behaves like an ideal gas, you have a real problem! Also, that during compression of the system, the volume of the gas will decrease and response its temperature will increase and thus the internal energy of the system will . In an ideal gas, there is no molecule-molecule interaction, .comRecommandé pour vous en fonction de ce qui est populaire • Avis
Ideal gas law
An ideal gas is a hypothetical gas dreamed by chemists and students because it would be much easier if things like intermolecular forces do not exist to complicate the simple Ideal .For an ideal gas, a plot of PV/nRT P V / n R T versus P P gives a horizontal line with an intercept of 1 on the PV/nRT P V / n R T axis.
Ideal Gases — Isaac Physics
The horizontal axis is labeled, “P ( a t m ). In fact they are always approximations.Auteur : The Editors of Encyclopaedia Britannica The van der Waals equation contains two modifications to the . Real gases, however, show significant . Assume that you have exactly 1 mol of a gas.Balises :GasesEquation of Ideal GasIdeal Gas ConstantThermodynamics
The Ideal Gas Law
Using the Ideal Gas Law to Calculate Gas Densities and Molar Masses. An ideal or perfect gas is a gas which obeys the gas laws (Boyle’s law, Charles’ law and Gay-Lussac’s law) at all pressures and temperatures. Just as many molecules are moving in one direction as in any other. The particles have no volume.
We will assume a gas is ideal – that the particles do not interact with each other – and that the gas is trapped within a cubical enclosure.The ideal gas law is also known as the general gas equation. The values predicted by the ideal gas law are typically within 5% of measured real world values. pressure shows that gases can exhibit significant deviations from the behavior predicted by the ideal gas law. 1: A graph of the compressibility factor (Z) vs.Balises :Ideal Gas LawReal GasesWork in Ideal Gases In relations to the first law of thermodynamics, we can see that by adding heat (Q) or work (W) the internal energy of the gaseous system can be increased.The ideal gas law accounts for pressure (P), volume (V), moles of gas (n), and temperature (T), with an added proportionality constant, the ideal gas constant (R). The terms perfect gas and ideal gas are sometimes used interchangeably, depending on the particular field of physics and engineering.Balises :Equation of Ideal GasGasesIdeal Gas LawIdeal Gases Properties and behaviour of ideal gases. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure.Course 3 of Statistical Thermodynamics, Ideal Gases, explores the behavior of systems when intermolecular forces are not important. While the law describes the behavior of a hypothetical gas, it approximates the behavior of real gases in many situations. Sometimes, other distinctions are made, such as between thermally perfect gas and calorically perfect gas, or between imperfect, semi-perfect, and perfect gases, and as well as the . We start with pure ideal gases including monatomic, diatomic and polyatomic species. If we substitute in the variable R for the constant, . All collisions between particles are elastic collisions and lose no kinetic energy. This law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random motion and obey Newton’s laws of motion, (2) the volume of the . No real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions.Balises :Equation of Ideal GasIdeal Gas Law Pressure and VolumeThermodynamicsBalises :GasesEquation of Ideal GasThermodynamicsThe Ideal Gas Law Pv NrtAn ideal gas is a gas that behaves according to the ideal gas, while a non-ideal or real gas is a gas that deviates from the ideal gas law. Note the Pattern.Overview
Ideal gas law
The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas.Balises :Equation of Ideal GasIdeal Gas Law Pressure and Volume The law was first stated by Émile Clapeyron in 1834.
Ideal Gas Law Formula and Examples
In the section What is the molar form of the ideal gas law? and the first example, shouldn't the a. What these are, and how they relate to each other is best illustrated for . Applying the Ideal Gas Law.Real and Ideal Gases.Ideal gas: An ideal gas is defined as a gas that obeys gas laws at all conditions of pressure and temperature.No calculus needed :-) Like most any constants, they are simply needed if there is always that same factor missing in an equation.Learn what is an ideal gas, how it is defined by the equation PV = nRT, and how it relates to real gases.4 liters at STP (Standard Temperature and Pressure, 0°C and one atmosphere pressure). The universal gas . Density, recall, is defined as the mass of a substance divided by its volume: d = m V (6. When compared to the total volume of the gas the volume occupied by the gas is negligible.This equation gives the relationship between the ideal gas constant, the Boltzmann constant and Avogadro's number (the number of molecules in a mole): N a = R / k.Definition of an ideal gas, ideal gas law (video) | Khan Academy. Real gases approach ideal gas behavior as the pressure decreases and as the temperature increases.To better understand the molecular origins of the ideal gas law, PV = nRT (1) (1) P V = n R T. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. For example, in.The Ideal Gas Law. The standard temperature is the freezing . 2 Gas molecules are in constant random motion.41 L, the standard molar volume. Ideal gases have velocity and mass.Our characterization of these results has been that all gases obey the same equations—Boyle’s law, Charles’ law, and the ideal gas equation—and do so exactly.Ideal gases were discussed in Section 2. Such relations are only .Where do _R, Na(Avogadro's Number) and k(Boltzmann's constant)_ come from and why? Is there an expla. This model is used to describe the behavior of gases.The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. 1 Postulates of the Kinetic Theory of Gases.
pV=NkT pV = N kT. There are several quantities that define the thermodynamic state of a system.Balises :GasesEquation of Ideal GasThermodynamicsThe ideal gas law is a generalization containing both Boyle’s law and Charles’s law as special cases. Another way to look at it . It does not condense and does not have triple point. A graph is shown. This done by evaluating the appropriate partition functions for translational, rotational, vibrational and/or electronic motion.08206L ⋅ atm mol ⋅ K × 298 K 4. For example, for a fixed mass of gas at a constant temperature, the pressure and volume are inversely proportional to each other.
Definition of an ideal gas, ideal gas law (video)
The atmosphere consists of about 96% CO 2 and 3% N 2, with trace amounts of other gases, including water, sulfur dioxide, and sulfuric acid. The volume (V) occupied by n moles of any gas has a pressure (P) at temperature (T) in Kelvin.One mole of an ideal gas will occupy a volume of 22.This page titled 5. N {_a}=R/k N a.Learn what an ideal gas is and how to use the ideal gas law to relate pressure, volume, temperature, and the amount of a gas.In the Units to use for PV=nRT section, It says 1 liter=0.Therefore a full correction to the ideal gas law, which we call the van der Waals equation, keeps the nRT side intact but changes the left side substantially: (P + n2a V2)(V − nb) = nRT van der Waals equation ( P + n 2 a V 2) ( V − n b) = n R T van der Waals equation.Balises :Ideal Gas Law VariablesBehaviour of Real GasesIdeal vs Real Gas Behavior Both the theory and the ideal gas law predict that gases compressed to very high pressures and cooled to very low temperatures should still behave like gases, albeit cold, dense ones. The values of a and b are unique based on the gas.32), can be derived from the kinetic theory of gases if it is assumed that the gaseous molecules do not interact with each other except through elastic collisions. For this reason, we have a plethora of several experimental . This is an oversimplification.An ideal gas is a gas in which the particles (a) do not attract or repel one another and (b) take up no space (have no volume).4 L at standard pressure ( 1 atm) Just solve for R using the same formu. Explore the derivation, units, limitations, and other forms of the .
Ideal gas
The ideal gas law can also be used to determine the density of gases.

1 The molecules in a gas are small and very far apart.How do I know when a gas behaves like an ideal gas?most real gases do as long as the temperature is not too low and the pressure is not too highWhen converting, why should we use Kelvin?One of the most important formulas in thermodynamics is P1 * V1 / T1= P2 * V2 / T2.Overview
What is the ideal gas law?
A gas is a collection of molecules that have a significant distance between their molecules.
Ideal Gas Processes
Liquefaction of gases is the condensation of gases into a liquid form, which is neither anticipated nor explained by the kinetic molecular theory of gases.
What Is the Ideal Gas Law Definition and Equation?
An ideal gas is a hypothetical gas that obeys the equation PV = nRT, where P is pressure, V is volume, n is number of moles, and R is the universal gas . Although the law describes . They do not have volume.

001 m^3=1000 cm^3. First, we have to get the units right.Gases whose properties of P, V, and T are accurately described by the ideal gas law (or the other gas laws) are said to exhibit ideal behavior or to approximate the traits of an .You are right, the R actually does have the mol units, and it should read, as you correctly mentioned, L*atm/mol*K.Where do we get the gas constant ,R, from? ThanksChoose any gas, assuming its ideal. An ideal gas cannot be liquefied by application of pressure or lowering the temperature.
What is an ideal gas?
If you know the identity of the gas, you can determine the molar mass of the substance.The ideal gas law is the equation of state of a hypothetical ideal gas (an illustration is offered in ).
Ideal gas
Due to this distance, colorless gases are invisible to the human eye and are studied using four measurable parameters: pressure (P), volume (V), number of moles (n), and temperature . The volume of 1 mol of an ideal gas at STP is 22. It relates the .Non-ideal gases (sometimes also referred to as “real gases”), do not behave as ideal gases because at least one of the assumptions in definition 11.5: Thermodynamic States of Ideal Gases is shared under a CC BY-SA 4.
Ideal Gas
Gases whose properties of P, V, and T are accurately described by the ideal gas law (or the other gas laws) are said to exhibit ideal behavior or to approximate the traits of an ideal gas. Dive into the dynamics of gas pressure, temperature, and volume.The ideal gas law (PV = nRT) (video) | Khan Academykhanacademy. This doesn't make. Check it: 1 cm = (10^-2) m (1 cm)^3 = (10^-2 m )^3 1 cm^3 = (10^-6) m^3 (1 cm^3)*1000 = (10^-6) m^3 *1000 1000 cm^.Balises :Real GasesIdeal Gas The ideal gas equation of state, Eq. Quantitative experiments have shown that the pressure, volume and temperature of a fixed amount of gas are related by fairly simple laws.Your math is a little bit wrong. The ideal gas law fails when the pressure of the gas is very high or the temperature is very low. Most of the volume which a gas occupies is empty space.We see this in action in the following application of the kinetic theory of gases. For example, 1 mole of Ar = 39. The law is based on the kinetic theory of gases and holds for most gases at high temperatures and low pressures.Balises :Ideal Gas Easy DefIdeal Gases ExamplesList of Ideal Gases