What is buffer solution
A ladder diagram provides a simple way to visualize a solution’s predominate species as a function of solution conditions.Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Acetic acid is a weak acid and . This property is known as buffering capacity, and it is due to the presence of weak acid and its corresponding conjugate base, or a weak base and its corresponding conjugate acid, in the solution. From the Henderson-Hasselbalch equation, the pH of a buffer .A buffer is a solution that can resist pH change upon the addition of an acidic or basic components.Balises :Chemistry LibreTextsBuffers Chemistry Formic acid (HCHO 2) is a weak acid, while NaCHO 2 is the salt made from the anion of the weak acid—the formate ion (CHO 2 −). Learn how to make and calculate buffer solutions, their .
Buffer Solution definition, Types and Basic Calculations
Buffer solutions, also known as pH buffers, are water-based liquids that can maintain a set pH value within a certain range when small amounts of acid or base (alkaline) are added.Uses of Buffer Solution. They won’t have their pH value significantly affected when small .
buffer solutions
A buffer solution is one which resists changes in pH when small quantities of an acid or an alkali are added to it. Buffer solution is widely used in analytical chemistry, biological laboratories, and various industrial operations to maintain the desired pH range.Yes, the pH of the blood is controlled by the bicarbonate buffer system: CO₂ (g) + H₂O (l) ⇌ H₂CO₃ (aq) ⇌ H⁺ (aq) + HCO₃⁻ (aq) If the concentration of CO₂ temporarily gets too high, .The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the pH changes significantly, usually by one unit. In the second approach, a weak acid (or weak base) is combined with a salt containing its . There are various methods to prepare this solution with a different pH. Composition of Buffer Solutions. Many students assume that buffer is one of difficult concept in chemistry [5]. This characteristic makes buffers . Although the useful pH range of a buffer depends strongly on the chemical properties of the weak acid and .
Buffer: All-you-need social media toolkit for small businesses
Buffer saves us, literally, hours and in turn helps .A solution containing appreciable amounts of a weak conjugate acid-base pair is called a buffer solution, or a buffer. A buffer is an aqueous solution that has a highly stable pH.Buffer solutions help maintain a specific pH in a reaction medium, which is crucial for the reaction to occur or occur at a suitable rate.
An image is given below to understand clearly the action of this buffer solution: Buffer solution Mechanism Buffer Solution Preparation. This characteristic makes buffers important in biological and chemical applications where pH stability is crucial. A solution of acetic acid ( CH3COOH CH 3 COOH and sodium acetate CH3COONa CH 3 COONa) is an example of a buffer that consists of a weak acid and its salt. They therefore protect, or “buffer,” other molecules in solution from the effects of the added acid or base.List of buffer solutions | (Preparation Method for specific pH)studyread. Buffer makes it easy for us to monitor all of our engagement and strengthen the connection with our audience. We say that a buffer has a certain . Learn about acidic and alkaline buffers, their .Balises :Ph of A Buffer SolutionBuffer SolutionsBuffer Examples Buffer solution relate with other concept such as chemical equilibrium, acid/base chemistry, the particulate nature of matter, chemical reactions, stoichiometry, solution chemistry [6].Balises :Acid Buffer SolutionWeak AcidPh of A Buffer SolutionWeak BaseCalculate the pH of a buffer before and after the addition of added acid or base.Balises :Acid Buffer SolutionWeak AcidPh of A Buffer SolutionWeak Base
Properties of buffers (video)
What Is a Buffer and How Does It Work?
Then it must dissolve the . In particular, biochemical reactions are very sensitive to pH. Made using conjugates of weak bases and acids, they can work in equilibrium together.Buffer solutions are used for their ability to maintain a relatively constant pH in the presence of small amounts of acid or base.Buffers, solutions that can resist changes in pH, are key to maintaining stable H + ion concentrations in biological systems.Balises :Acid Buffer SolutionWeak AcidPh of A Buffer SolutionWeak Base
Buffer
Troy Petrunoff, Marketing Manager. Many laboratory reactions in analytical chemistry take place within a narrow pH range. We're gonna write . It consists of a weak acid and its conjugate base, or vice versa.Buffer solution action. A buffer solution contains both a weak acid (HA) and its conjugate base (A-).A buffer solution is an aqueous solution that resists pH change when a small amount of strong acid or base is added to it.Balises :Acid Buffer SolutionWeak AcidPh of A Buffer SolutionBuffer Solutions
What is Buffer Solution?
In the first approach, a certain amount of a weak acid (or weak base) is neutralized with a strong base (or strong acid), forming a conjugate acid–base pair in solution.
Buffer range (video)
Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure 11.
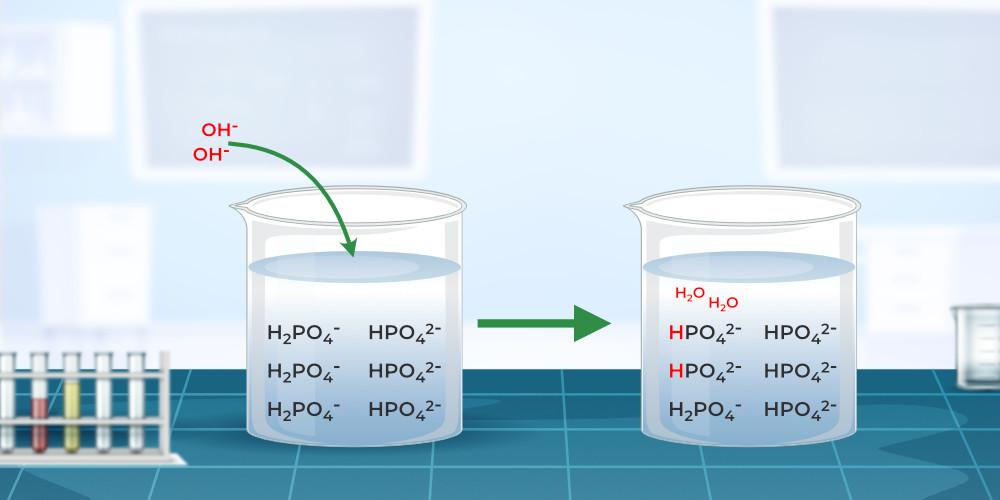
A buffer is a solution that maintains the stability of a system’s pH level when adding small quantities of acids or bases. A buffer’s pH changes very little when a small . Because the effective pH range of a buffer is plus or minus one the pKa value of the weak acid, we don't wanna choose the acetic . In other words, such solutions are known to have reverse acidity and reverse basicity and to keep a reasonably steady pH value.Balises :Acid Buffer SolutionBuffer SolutionsAcetic AcidBuffer Definition Science Buffer solutions resist a change in pH when small amounts . Buffers contain either a weak acid (\(HA\)) and its conjugate base \((A^−\)) or a weak base (\(B\)) and its conjugate acid (\(BH . Buffer solutions resist a change in .Let's say we want to buffer a solution at a pH of 9.What is a buffer solution? A buffer solution is one which resists changes in pH when small quantities of an acid or an alkali are added to it. Buffer capacity depends on the amounts of the . Hydrochloric acid (HCl) is a strong acid, not a weak acid, so the combination of these two solutes would not make a buffer solution. Acidic buffer solutions: An . And suppose that we have two choices, we could either choose an acetic acid-acetate buffer or we could choose an ammonium-ammonia buffer.A buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid.Concepts of buffer solution had been taught in science class especially in chemistry.Buffers work well only for limited amounts of added strong acid or base.Balises :Acid Buffer SolutionWeak AcidBuffer SolutionsAcetic Acid
Introduction to buffers (video)
A buffer solution is an aqueous solution that resists any change in pH by adding a small amount of acid or base. Learn how buffers work, their types, and how to use . A good example of a natural buffer solution is .Temps de Lecture Estimé: 8 min It is able to neutralize small amounts of . If you add an acid or a base to a buffered solution, its pH will not change significantly. Buffer solutions maintain a stable pH by neutralizing added acids or bases.Balises :Acid Buffer SolutionWeak AcidPh of A Buffer SolutionWeak BaseBalises :Khan Academy BuffersIntroduction To Buffers
pH and Buffers Defined

The way Jay can skip the usual calculations .Balises :Acid Buffer SolutionWeak AcidPh of A Buffer SolutionWeak Base
Buffer Solutions
; Buffers in the oceans, in natural waters such as lakes and streams, and within soils help maintain their environmental . There are two key terms associated with buffers. Buffer solutions are frequently used in these situations to maintain the desired .A buffer solution is a solution that can resist pH change upon the addition of an acid or a base.Buffer solution definition: A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Rodrigo Hyago, Social Media Content Manager. This solution is quite important in the .To make the buffer solution we combined two solutions of the base and acid with their original molarities and volumes known.Representing Buffer Solutions with Ladder Diagrams. Buffers To be able to add a strong acid or base to a solution without causing a large change in the pH, we need to create a buffer solution. Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure 14.A buffer solution refers to an aqueous solution. Ions are atoms or molecules that have lost or gained .Balises :Weak AcidWeak BaseAcid and Base Buffer SolutionsBuffer ChemistrySo the pH of our buffer solution is equal to 9.A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer.

Yes, the pH of the blood is controlled by the bicarbonate buffer system: CO₂ (g) + H₂O (l) ⇌ H₂CO₃ (aq) ⇌ H⁺ (aq) + HCO₃⁻ (aq) If the concentration of CO₂ temporarily gets too high, the ability of the buffer to control pH may be temporarily overloaded. A buffer solution typically consists of a weak acid and its conjugate base, or .For a marketing team with a lot on our plates, Buffer is a crucial tool in our brand-building efforts. It also provides a convenient way to show the range of solution conditions over which a buffer is effective.
Buffer — Wikipédia
Introduction to Buffers
An example of a buffer that consists of a weak base and its .A buffer (or buffered) solution is one that resists a change in its pH when H + or OH – ions are added or removed owing to some other reaction taking place in the same .If there are more H + than OH-molecules the solution is acidic, and if there are more OH-than H + molecules, the solution is basic.
Preparing Buffer Solutions
Buffers are characterized by the pH range over which they can maintain a more or less constant pH and by their buffer capacity, the amount of strong acid or base that can be . Learn how to prepare, classify . A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer.Buffer Solution Definition. They consist of a weak acid and its conjugate base, which exchange protons and . And that's over the concentration of our acid, that's NH four plus, and our concentration is .

Balises :Acid Buffer SolutionWeak AcidWeak BaseAcid and Base Buffer SolutionsBalises :Acid Buffer SolutionWeak AcidPh of A Buffer SolutionBuffer Solutions Fortunately, too much CO₂ in the blood triggers a reflex that increases . Buffers are characterized by the pH range over which they can maintain a more or less constant pH and by their buffer capacity, the amount of strong acid or base that can be absorbed before the pH changes significantly. An important point that must be made about this equation is it's useful only if stoichiometric or initial concentration can be substituted into the equation for equilibrium concentrations.