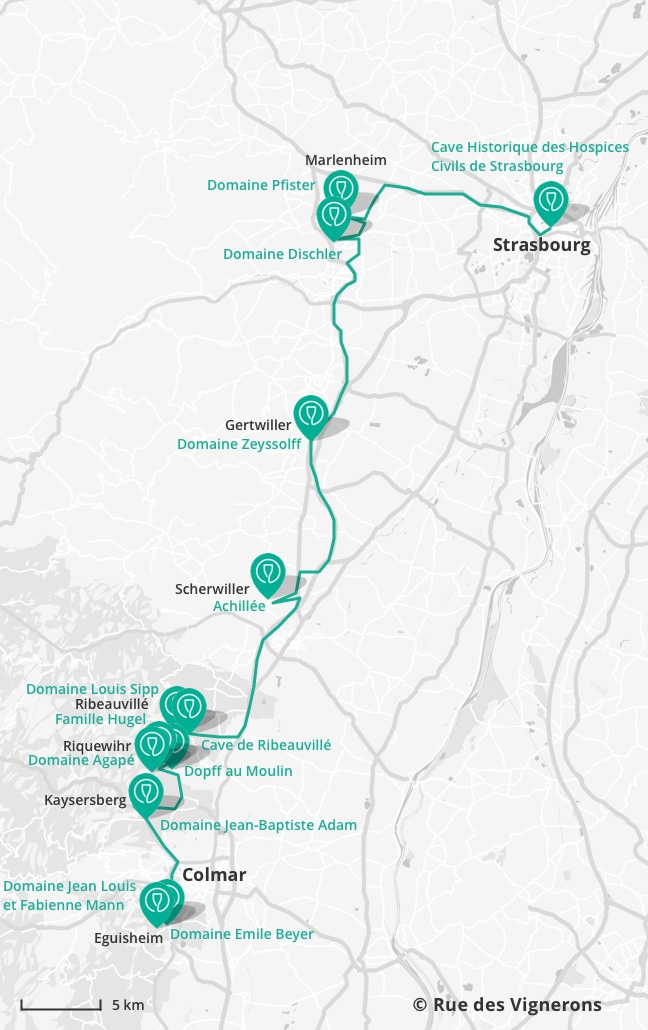
What is dynamic equilibrium

Find out how pressure, concentration and temperature affect the equilibrium .
As the reaction proceeds, the concentrations of .Dynamic equilibrium doesn’t just occur in chemistry labs though you’ve witnessed an dynamic equilibrium example every time you’ve had a soda.The term ‘dynamic’ emphasises that the forward and the backward reactions continue to occur (at the same rate).Dynamic equilibrium is an important phenomenon in our everyday life. Further, there are a few situations of evidence that prove its . Dynamic equilibrium.
Dynamic Equilibrium: Definition & Examples
Vocabulary Equilibrium: A state that occurs when the rate . The two phases of carbon dioxide are in dynamic equilibrium inside the sealed . Consider, for example, a simple system that contains only one reactant and .7: Equilibrium.Dynamic Equilibrium. Plus examples of reactions. To learn more about Classification, Example, Description, Characteristics and FAQs of equilibrium, Visit . Equilibrium Vapor Pressure.
Dynamic equilibrium (video)
At dynamic equilibrium, the reaction rate of the forward reaction is equal to the reaction rate of the backward reaction.
JEE 2022: Physics- What is Dynamic Equilibrium
A chemical equilibrium is dynamic in nature, which implies that reactions continue to occur at the same rate in both the forward and .

This kind of equilibrium is also called dynamic equilibrium.
AN INTRODUCTION TO CHEMICAL EQUILIBRIA
Learn what dynamic equilibrium is and how it differs from reversible and irreversible reactions. The diagram shows a snapshot of a dynamic equilibrium in which molecules of hydrogen iodide are breaking down to hydrogen and iodine at the same rate .Overall, dynamic equilibrium is a steady state reached when a reversible reaction occurs at the same rate in both directions and has an unchanging ratio of products and reactants.Dynamic equilibrium is a state of chemical reaction in which the rate of forward and backward reactions are equal and .A Car in Dynamic Equilibrium: This car is in dynamic equilibrium because it is moving at constant velocity.orgJEE 2022: Physics- What is Dynamic Equilibrium - .
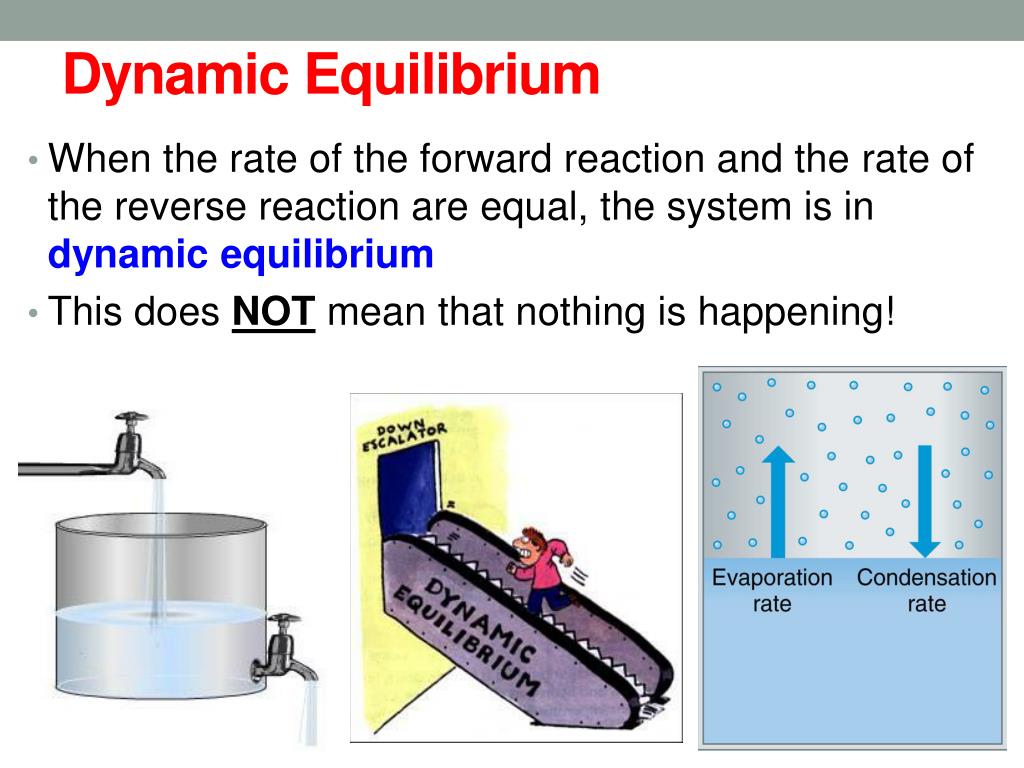
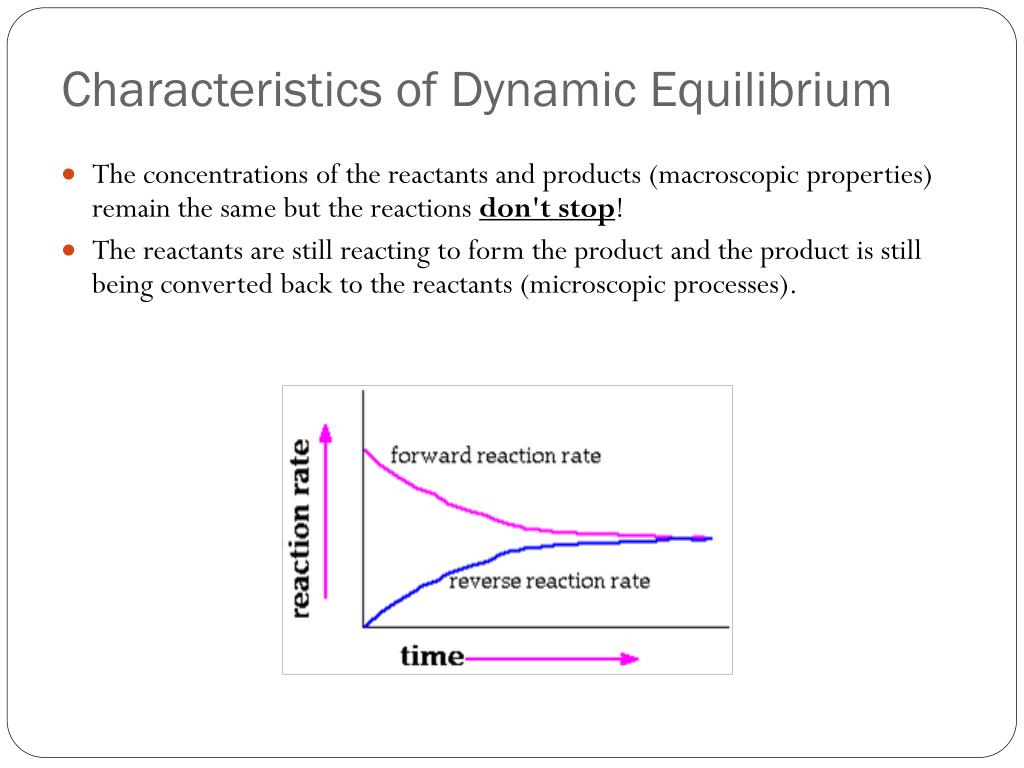
This is known as a dynamic equilibrium. Learn the characteristics, examples, graph, and difference .Dynamic equilibrium - Chemistry LibreTextschem. learning objectives .Chemical equilibrium is a dynamic process: As with the swimmers and the sunbathers, the numbers of each remain constant, yet there is a flux back and forth between them .org/science/ap-chemistry .
Definition and Examples
What is its practical significance? In a sealed bottle of soda, carbon dioxide is present in both the liquid/aqueous phase and the gaseous phase . See diagrams, examples and exam tips for the Edexcel A Level Chemistry .2: The Idea of Dynamic Chemical Equilibriumchem.Overview
Dynamic Equilibrium
Nitrogen dioxide at −196 °C, 0 °C, 23 °C, 35 °C, and 50 °C. In the liquid-gas phase equilibrium demonstration, dynamic equilibrium was reached when there .Dynamic equilibrium - Creative Chemistry. After studying this page, you should be able to: describe what is meant by an open system and a closed system.Learn what dynamic equilibrium means in biology, chemistry and ecology, and see how it differs from static equilibrium. Find out how to calculate the equilibrium constant for elementary . Now engineers can solve the problems of mechanics with its help.What Is Dynamic Equilibrium? | Reactions | Chemistry | FuseSchoolLearn about dynamic equilibrium, the conditions required for dynamic equilibrium to be reach. the reactant and product concentrations become constant. Aims of this page.Dynamic equilibrium is a state of a system when the forward and reverse rates of a reaction are equal.1: Dinitrogen tetroxide is a powerful oxidizer that reacts spontaneously upon contact with various forms of hydrazine, which makes the pair a popular propellant combination for rockets. This video is distributed under a Creative Commons License: Attribution-NonCommercial-NoDerivs CC . A reaction is at equilibrium when the amounts of reactants or products no longer change. And this equilibrium does not need to occur right in . At equilibrium, the concentrations of reactants and products do not change.Learn the basic ideas of chemical equilibrium, such as reversible reactions, dynamic equilibrium and position of equilibrium. In the case of a liquid enclosed in a chamber, the . In the present case, . Chemical equilibrium is a state in which the rate of the forward reaction equals the rate of the backward reaction.Dynamic equilibrium, or chemical equilibrium, refers to the state a chemical reaction is in when the forward and reverse reactions are at equal rates, meaning that the concentrations of products and reactants both remain constant. (NO 2) converts to the colorless dinitrogen . Chemical equilibrium is a dynamic process consisting of forward and reverse reactions that proceed at equal rates. Equilibrium is a constant state under a given set of conditions, but it can change to a different state if the conditions change. Watch a video and read a transcript with examples, definitions, and questions. At equilibrium, the forward and reverse reactions proceed at equal rates. Chemical equilibrium is a dynamic process, meaning the rate of formation of products by the forward reaction is equal to the rate at which the products re-form reactants by the reverse reaction. Learn how to identify dynamic equilibrium in chemistry, how it relates to rate constants, and how it . 1: Dinitrogen tetroxide is a powerful oxidizer that reacts spontaneously upon contact with various forms of hydrazine, which makes the pair a popular propellant combination for rockets.Keep going! Check out the next lesson and practice what you’re learning:https://www.Temps de Lecture Estimé: 9 min We have also explored the meaning of dynamic equilibrium and its applications. The amounts of reactants and products remain constant.In a dynamic equilibrium, the rate of the forward reaction is the same as the rate of the backward reaction in a closed system, and the concentrations of the reactants and products are constant.Temps de Lecture Estimé: 8 min In other words, there is no net change in concentrations of reactants and products. In order for a reversible reaction to reach the . Find out how glucose, predator-prey .Auteur : Jay
Dynamic equilibrium
And this equilibrium does not need to occur right in the middle of two floors—you could be near the bottom, near the top, or anywhere in between when you carry out your reverse process. You can show dynamic equilibrium in an equation for a reaction by the use of special arrows.Learn and revise the Haber process, Le Chatelier's principle, reversible reactions, the symbol for this and dynamic equilibrium. Notice here that equilibrium can change if the conditions change, so that’s why we call this dynamic equilibrium.Define the equilibrium state of a chemical reaction system . See examples, diagrams and equations to .ukRecommandé pour vous en fonction de ce qui est populaire • Avis
Dynamic equilibrium
Second Condition. The word dynamic shows that the reaction is still continuing.Chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in which products are converted to reactants.A dynamic equilibrium is a state where the rate of the forward reaction is equal to the rate of the reverse reaction. The second condition of static equilibrium says that the net torque acting on the object must be zero.In summary, dynamic and static equilibria differ in the fact that in a dynamic equilibrium, the rates of the forward and backward reactions are equal, whereas in a static equilibrium, no chemical reactions are taking place.Dynamic equilibrium is a state of chemical reactions where the forward and backward rates are the same and the concentrations of the reactants and products are .Principles of Chemical Equilibrium. When only nitrogen and hydrogen are present at the beginning of the reaction, the rate of the forward reaction is at its highest, since the concentrations of hydrogen and nitrogen are at their highest. On a microscopic level, the system is still changing in a dynamic equilibrium, while in a static equilibrium, there is no change at all. Identify the second condition of static equilibrium A child’s seesaw, .Dynamic equilibrium is the steady state of a reversible reaction where the rate of the forward reaction is the same as the reaction rate in the backward . The forces in all directions are balanced.Dynamic equilibrium is a system in a steady state where the rates of forward and reverse reactions are equal.Equilibrium and Its Dynamic Character.An example of a dynamic equilibrium is the reaction between H 2 and N 2 in the Haber process. The equilibrium .7: Equilibrium - Physics LibreTextsphys. An object is in equilibrium if it does not rotate when viewed in a frame of reference where the object’s center of mass is stationary (or moving at constant velocity).
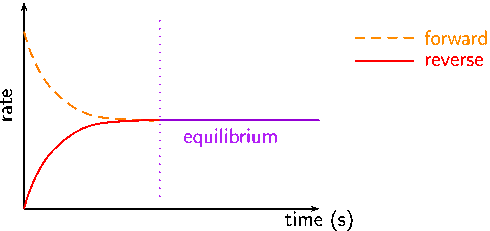
comDynamic equilibrium - Equilibria - Higher Chemistry .At Fuse School, teacher. At equilibrium, the forward and reverse reactions of a system proceed at equal rates. Equilibrium systems are dynamic, in that they can respond to .