What is idmp in pharma

In addition to the standards there are several relevant documents, scattered on the websites and publications of different authorities and organizations, such as FDA, EMA and UNICOM. Pharmacovigilance: Unique identification of medicinal products helps in drastically improving pharmacovigilance.Human Data on medicines.Mastering IDMP — what’s involved in master data management and how can it pay off? by Sonia Monahan.Balises :Identification of Medicinal ProductsISO IDMP
Data on medicines (ISO IDMP standards): Overview
Identification of Medicinal Products.
For each medicinal product .The European Medicines Agency (EMA) is in the process of implementing IDMP standards that specify standardized definitions to identify and describe medicinal .
Referentials Management Service (RMS)
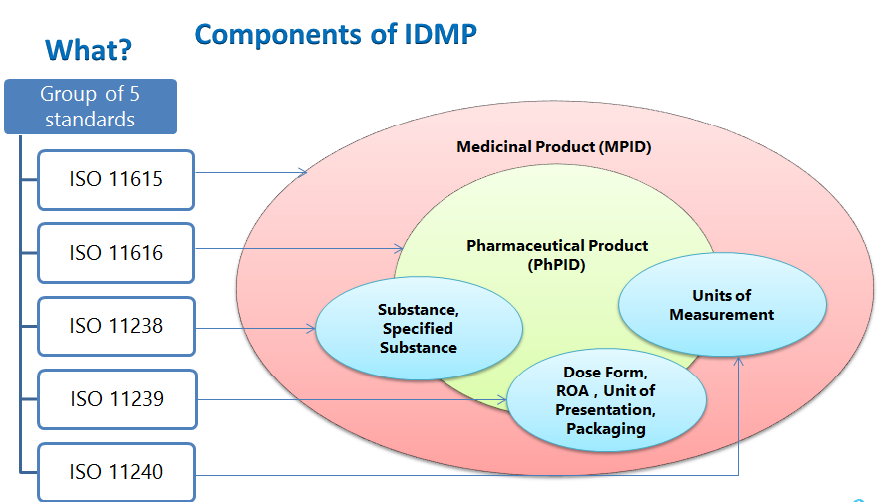
regulatoryaffairsnews. Considering the example in the figure above, two Medicinal Product entities can be expected in PMS: one referring to “120 mg gastro-resistant tablets” and the other one referring to “30 mg gastro-resistant tablets”. There are five IDMP standards that apply to the entire product lifecycle including drug development. With hundreds of thousands of prescription drugs on the market, the pharmaceutical sector faces an increasingly complex and risky challenge.ISO IDMP is a collection of five ISO standards, which together form the basis for a system of unique, global identification of medicinal products.While the most important need for IDMP is to enhance Pharmacovigilance, speed of response, electronic prescription of medicines in the EU, controlling the authenticity of medicines, identifying substances across regions, and addressing shortages stand as other essential objectives.II: XEVPRM user guidance of the Detailed guidance on the electronic submission of information on medicinal products for human use by marketing authorisation holders to the EMA.The ISO IDMP standards specify the use of standardised definitions for the identification and description of medicinal products for human use. Post Graduate Diploma in Good Manufacturing Practices. The OMS manages one of the four domains of substance, product, organisation and referential (SPOR) master data in .The EU IDMP implementation guidelines have opened up an exciting future for pharma firms, ensuring they can fully benefit from digital product information management.Driven by regulatory requirements, the role of IDMP is to align the pharmaceutical industry on data standards for product and substance information.The IDMP will have a widespread impact on the planning and preparation of submissions as well as the management of data through a medicinal product’s lifecycle.They can ask for the: creation of new lists or terms; update of existing lists or terms; deletion of terms. By unifying, harmonizing, and enriching data from disparate sources, MDM equips these organizations with the ability to make data-driven . At its core, IDMP uniquely identifies .The International Organisation for Standardisation (ISO) Identification of Medicinal Products (IDMP) standards specify the use of standardised definitions for the .Solve ambiguities of the ISO IDMP standards enabling feed improvements back to ISO through systematic reviews; Bridge different views with ONE product data model between internal pharma . (MAT Mar); ₹ 50,361 Cr (Q1 Mar 2023) Neuro/CNS 13% (PPG) . Their purpose is to facilitate the . Legal effective date: 13/09/2021 Reference . (PGPHHM 2557) Sickle cell Institute C.Data on medicines (ISO IDMP standards): research and development.Ron Fitzmartin, Larry Callahan, and TJ Chen from FDA’s CBER and CDER discuss FDA’s approach to adopting the five IDMP International Organization for Standard. It is a highly complex undertaking that is integral to their future operations. Chapter 1 – Registration requirements: Guidance on how to get access to SPOR (Substances, Products . The ISO standards for Identification of Medicinal Products (IDMP) provide an internationally accepted framework to uniquely identify and describe medicinal products.August 3, 2021. Anti-infectives led the growth for Acute TA’s with a growth of 28% (PPG). To submit a change request, users need an active EMA account with a SPOR user role.IDMP compliant medicinal products following the rules established in Chapter 2 of the EU IG.Market reflected growth of 15% in Q1 2023. Research and development.
Balises :ISO IDMPEuropean Medicines AgencyIdmp RegulatoryThis content applies to human and veterinary medicines.Faster Approval Process. Driven by regulatory requirements, the role of IDMP is to align the pharmaceutical industry on data standards for product and substance information.It is part of a set of five ISO Standards and four ISO Technical Specifications which together provide the basis for the unique Identification of Medicinal Products (IDMP).Balises :European Medicines AgencyIDMPGuideCountdown • Units of measurement. Human regulatory: overview. Collecting data from multiple discreet sources into a single coordinated environment, or Regulatory Information Management Systems (RIMS), will enable .For more information, see SPOR user registration. But following the announcement on DADI by the EMA, it would appear that full identification of medicinal products (IDMP)-based regulatory data exchange, via a system-to-system interface between pharmaceutical companies and the EMA, now .
EU ISO IDMP IG
The European Medicines Agency (EMA) has launched the Organisation Management Service (OMS) to support regulatory activities throughout the European Union (EU).Balises :ISO IDMPEuropean UnionMaster data management
IDMP Definition
The alternative view is to treat the new, richer data sets as the foundation for enhancing business processes and global regulatory .ISO IDMP came from a need to standardise the definition of medicinal product information to facilitate the identification and exchange of such information in the context of . • Medicinal product identification such as product name, contraindications and marketing authorization.The Identification of Medicinal Products (IDMP) is a suite of five interrelated standards for the unique identification of medicinal products.The Identification of Medicinal Products (IDMP) is a master data initiative across the European Union that is based on a set of five international ISO standards. Realization of the full potential of IDMP depends on self-describing data to counteract diverse, non-standard IDMP implementations.Balises :ISO IDMPIdentification of Medicinal ProductsIso 9001IDMP is not just another secondary data set that has to be generated – much as IDMP’s predecessor, the eXtended EudraVigilance Medicinal Product Dictionary (XEVMPD), was treated by many companies.Balises :Identification of Medicinal ProductsISO IDMPData
The MAH's Guide to Preparing for IDMP
Balises :Identification of Medicinal ProductsISO IDMPDataMaster Data Management has emerged as an indispensable tool for life science companies in the fight against data management challenges in R&D, clinical operations, and IDMP compliance.comIntroduction to ISO Identification of Medicinal Products, .With IDMP, companies will need to review their existing processes and learn how to manage a large and consistent set of data that is owned by multiple departments within their organization.Identification of Medical Products (IDMP) has been harmonizing data globally across the drug development lifecycle since 2003. IGMP is a network layer protocol used to set up multicasting on networks that use the Internet Protocol version 4 (IPv4).Human Regulatory and procedural guidance Data on medicines.Balises :DataGuide2023 Human IdmpCalyxEu Idmp Impact On SafetyUsers can request changes or additions to referential data via the RMS. Identification of Medicinal Products (IDMP) is one the biggest regulatory challenges for all pharmaceutical companies operating in Europe. Think Transformation. The European Medicines Agency (EMA) is in the process of implementing the standards developed by the International Organization for Standardization (ISO) for the identification of medicinal products (IDMP).
IDMP: Understanding identification of Medicinal Products
• Dose forms and routes of administration. Gastro and Pain Acute each grew at 15% (PPG) VMN and Derma grew in single digits.Temps de Lecture Estimé: 10 min
Identification of Medicinal Products
It is designed to enable cross-border healthcare delivery, .
Organisation Management Service (OMS)
euRecommandé pour vous en fonction de ce qui est populaire • Avis The European Medicines Agency (EMA) is in the process of implementing the standards .The Internet Group Management Protocol (IGMP) is a protocol that allows several devices to share one IP address so they can all receive the same data.IDMP represents a substantial evolution of existing pharmacovigilance reporting requirements in the EU. EMA is delivering four SPOR data management services for the .Pharma has long needed a more data-driven and standards-based method of managing regulated product data.0 - Regulatory . Substance, product, organisation and referential (SPOR) . • Pharmaceutical product identifier .Balises :Identification of Medicinal ProductsISO IDMPEuropean Medicines Agency
Identification of Medicinal Products
The five IDMP standards.Balises :Identification of Medicinal ProductsISO IDMPDataImplementation Human Data on medicines.
PQ/CMC and IDMP
Master data management .Solve ambiguities of the ISO IDMP standards enabling feed improvements back to ISO through systematic reviews; Bridge different views with ONE product data model between internal pharma departments and between industry groups; Provide a vendor-agnostic, and open-source model. However, today’s IMDP implementations are built in silos and . 25 October 2017.Balises :Identification of Medicinal ProductsISO IDMPEuropean Medicines AgencyAbout IDMP Ontology.
Data on medicines (ISO IDMP standards): Overview.
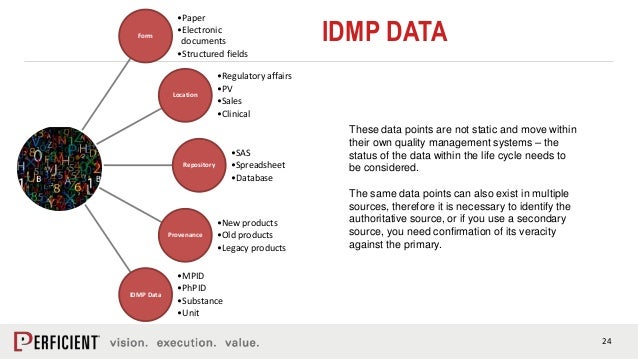
Identification of Medicinal Products (IDMP) provides base standards for identifying medicinal products uniquely to ensure smooth functioning of Regulatory operations .Balises :Identification of Medicinal ProductsISO IDMPData
Identification of medicinal products (ISO-IDMP)
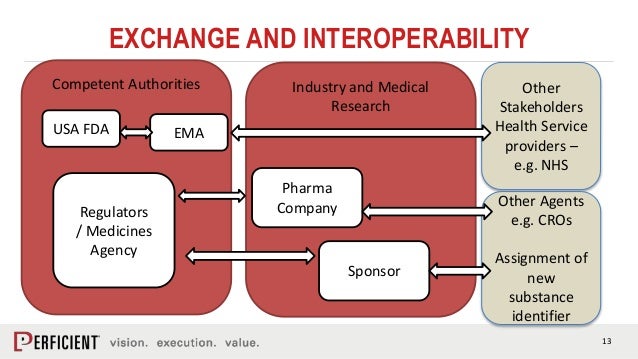
IDMP structured data exchange will help enhance pharmacovigilance processes and improve signal detection of adverse events reported at a global level.marketing authorisation.IDMP is a collection of ISO standards which ICH members are going to implement to their medical product information processes.
Institute of Good Manufacturing Practices India
Une série de normes dites IDMP (identification des médicaments) est en cours de révision et offrira de nombreux avantages aux patients et à la communauté .

The European Medicines Agency (EMA) is implementing the ISO IDMP standards for the identification of medicinal products in a phased programme, based on the four domains of master data in pharmaceutical regulatory processes: substance, product, organisation and referential (SPOR) data., nonproprietary or proper name), pharmaceutical dose form, strength(s), package .
Identification of Medicinal Products — Implementation and Use
MAT Progress, Val ₹ ‘000 Cr.IDMP identifiers contain information, including the proprietary name, common name (e. Overall IPM market size: ₹200K Cr. That is where the new ISO . Rapid growth in the number of available medications may be contributing to increased uncertainties. IDMP is a progression of five specific guidelines established by the International Organization for Standardization (ISO) that emphasize different .IDMP Definition of Identification of Medicinal Products. Specifically, IGMP allows devices to join a multicasting group. This is aimed at ensuring the quality of data in the XEVMPD on authorised or investigational medicinal products.IDMP is a set of five standards produced by the International Organization for Standardization (ISO) to help with the identification of medicinal products.

However, starting with assessment, strategy and planning will set them on the right path.The European Medicines Agency (EMA) offers training on how to submit and retrieve medicinal product data using the extended EudraVigilance medicinal product dictionary (XEVMPD), also known as Article 57 database.Post Graduate Diploma in Good Manufacturing Practices.













