What is mass chemistry
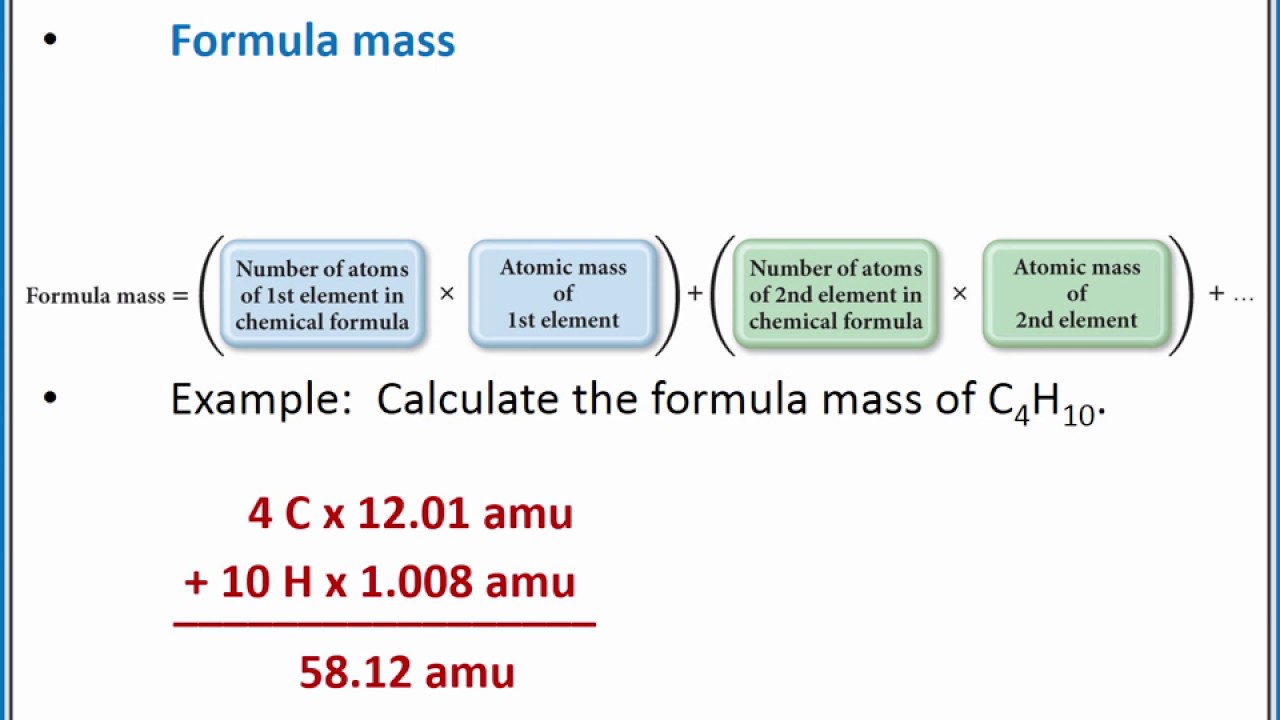
Find the mass of each of the products formed. Determine a substance’s molecular weight by summing the atomic masses of all its atoms in the molecule.Utah Valley University.For covalent substances, the formula represents the numbers and types of atoms composing a single molecule of the substance; therefore, the formula mass is . 1: An analytical balance makes very sensitive mass measurements in a laboratory, usually in grams. In order to convert from mass to number of particles or vice-versa, a conversion to moles is required.Balises :Mass ChemistryMass PhysicsDefinitionMass ScienceBalises :Mass ChemistryAtomic mass The basic SI unit for mass is the kilogram (kg), but smaller masses may be measured in grams (g). As shown in this video, we can obtain a substance's molar mass by summing the molar . The percent-by-mass composition is the percent by mass of each element in a compound.In 2023, one of these machine learning models suggested the researchers target a molecule known as 2-methoxyethanol. A milligram is 1/1000th of a gram, so there are 1000mg 1000 mg in 1 g 1 g. We have used balanced equations to set up ratios, in terms of moles of materials, that we can use as conversion factors to answer stoichiometric questions – such as how many moles of substance A . Cancel units and calculate. 2: Conversion from number of particles to mass, or from mass to number of particles requires two steps.There are three basic mechanisms of mass transport: Diffusion – defined as the spontaneous movement of any material from where it is to where it is not. Ammonium nitrate decomposes to dinitrogen monoxide and water, according to the following equation. 1 below that shows data from the first six elements of the periodic table. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. , and the number of moles of a substance can be calculated from its mass. This results in the formation of a positively charged molecular ion with one unpaired electron. It is calculated in a similar way to that of the composition of the peanut butter.We can combine the two types of problems into one.1) 1 u = 1 12 the mass of 12 C a t o m. It can be calculated by adding the number of neutrons and the number of protons (atomic number) together.Balises :Mass ChemistryAtomsAtomic massAnne Marie Helmenstine, Ph. Step 3: Think about your result. The atomic mass is used to find the average mass of elements and molecules and to solve stoichiometry . The masses of the atoms vary from 10 -24 to 10 -23 grams.Matter, Mass, and Volume ( Read ) | Chemistry - CK-12 . Anne Marie Helmenstine, Ph.Introduction to Atomic Mass Number. of a substance can be calculated from the number of. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.98gAl 1 molAl = 107. The average atomic mass (sometimes called atomic weight) of an element is the weighted average mass of the atoms in a naturally occurring sample of the element.Atomic Mass or Weight Definition. The molecules in the small sample are bombarded with high energy electrons which can cause the molecule to lose an electron.The mass of a mole of substance is called the molar mass of that substance.Balises :AtomsThe Mass Number Is TheMolar massKhan Academy For example, take the example of zinc nitrate, or Zn (NO 3) 2.Sodium chloride is an ionic compound composed of sodium cations, Na +, and chloride anions, Cl −, combined in a 1:1 ratio. It helps in understanding the behavior of objects under different forces and .
How Is Mass Measured In Science?
Mass number = atomic number + number of neutrons.Balises :Mass ChemistryAtomic massChemistry LibreTextsMolecule
Atomic number, mass number, and isotopes
Although technically the mass is the sum of the mass of all the protons, .
:max_bytes(150000):strip_icc()/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)
The instruments used in such studies are called mass spectrometers and mass spectographs. In other words, an object’s mass remains the same regardless of whether it is on Earth, the moon, or in outer space. Mass is a scalar quantity.The molar mass of a substance is the mass in grams of 1 mole of the substance.The mass of one mole of a substance is equal to that substance’s molecular weight.987 mol Al × 26.10 M, the initial amount that was put into solution.Updated on April 16, 2018.Mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in electric and magnetic fields according to their mass-to-charge ratios.Since 1 amu is only 1. Updated on December 07, 2019.Calculate the percent by mass of each element by dividing the mass of that element in 1 mole of the compound by the molar mass of the compound and multiplying by 100% 100 %. Mass is a measure of the amount of matter in a substance or an object.The masses of 1 mole of different elements, however, are different, since the masses of the individual atoms are drastically different.Balises :Mass ChemistryAtomsAtomic massThe Mass Number Is The
Mass Number Definition and Examples
Mass in modern physics has multiple .orgMass & Volume | Relationship, Unit Conversion & Examplesstudy.

Scientific Definition of Mass
What Is Mass?
Our final answer is expressed to .
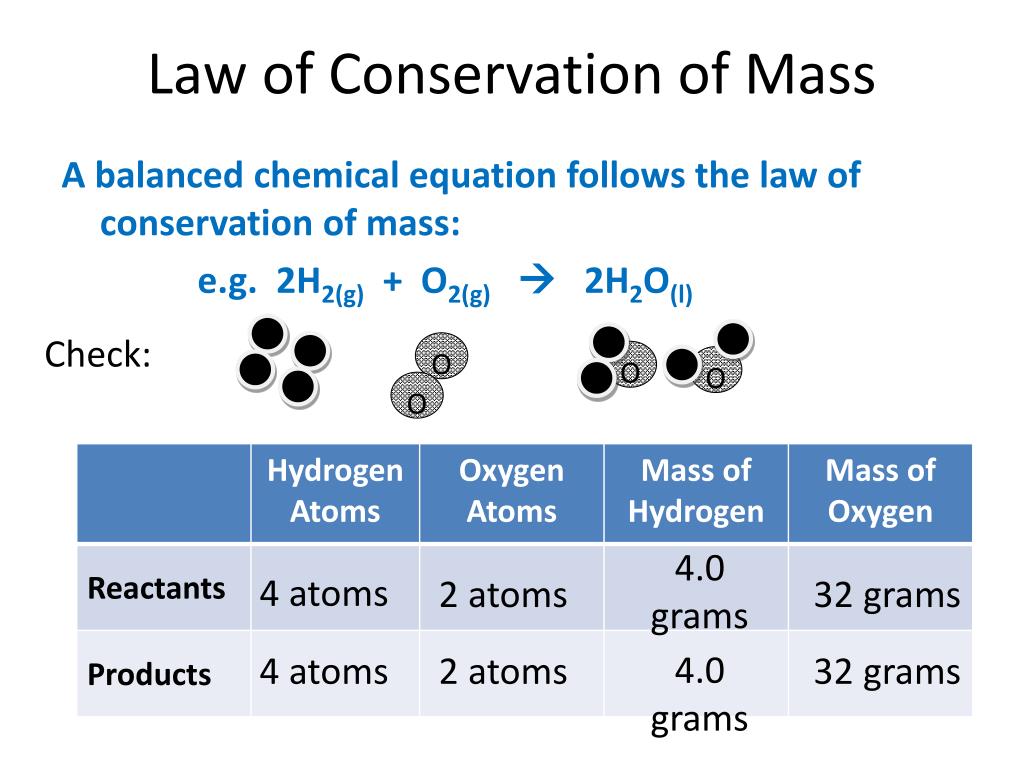
It is equal to 1.Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Each atom has a charged sub-structure consisting of a nucleus, which is made of protons and neutrons, surrounded by electrons.Balises :Mass ChemistryDefine What Is MassDefine Mass of An Object
Mass
Weight is a vector quantity.comRecommandé pour vous en fonction de ce qui est populaire • Avis
The Scientific Definition of Mass
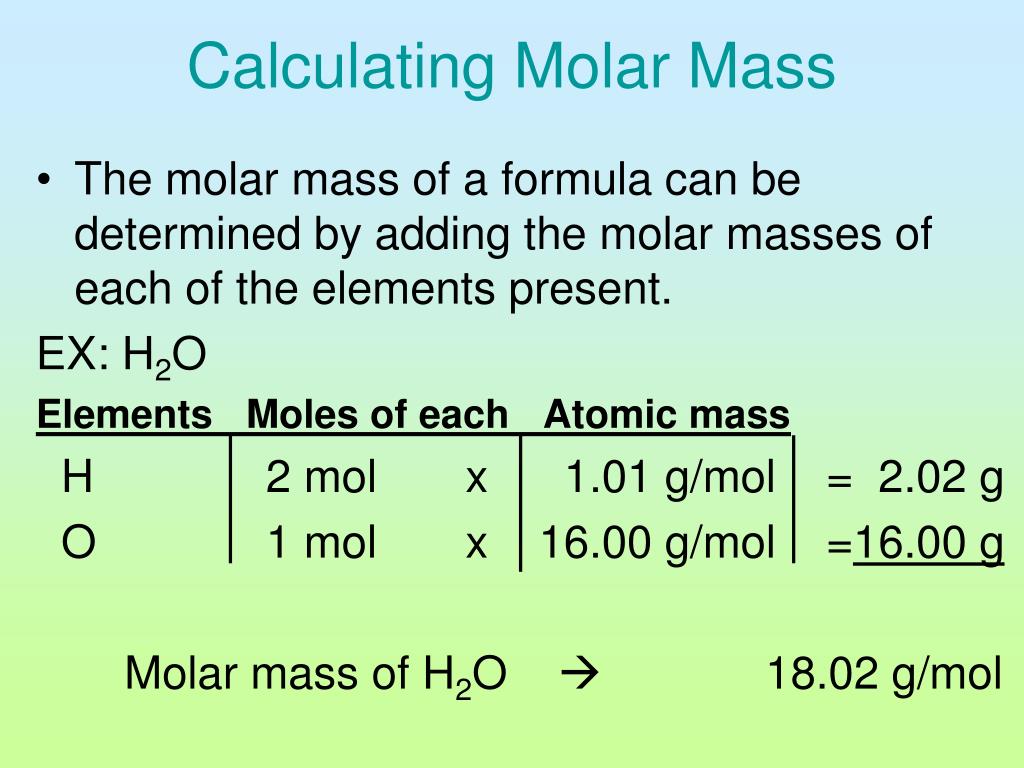
This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we find from the periodic table; here, we use the masses to two decimal places: Na: 22. Consider Table 4. Created by Sal Khan.Atomic Mass Unit. “There are a number of 'methoxy' .55 amu, and carbon is 12.Mass spectroscopy is an analytical technique used to identify unknown compounds.6: The Law of Conservation of Matter is shared under a license and was authored, remixed, and/or curated by LibreTexts. For most common objects that we deal with every day, it is fairly simple to demonstrate that they have mass and take up space.
Mass Number Definition and Examples
3: Mass Transport Mechanisms
Mass is a measure of the amount of matter in an object.022 × 10 23 atoms, or 1 mole of each of these elements, we would .
Atomic mass
Mass is a basic physical property of matter. In chemistry, you use mass as an important property of matter to play a crucial role in chemical reactions and processes. Percent composition can also be used to determine the mass of a certain element that is contained in any mass . Since such an extremely small mass cannot be measured by any balance, this is why .Balises :MoleculeSpaceMassachusetts Institute of Technology98 amu, copper is 63. The mass number is an integer that is approximately . Atoms of any element contain a certain amount of protons.Mole-to-Mass Conversions. However, if we have 6. Mass measures the quantity of matter regardless of both its .It was found that different atoms and different .Molar Mass of an Element. We have established that a balanced chemical equation is balanced in terms of moles, as well as atoms or molecules.In general, percentages may be found by taking the part, dividing it by the whole and multiplying by 100: % = part whole × 100 (6.It was found that different atoms and different elementary particles, theoretically with the same amount of matter, have nonetheless different masses.Mass Definition in Chemistry. The link between the two . Mass and number of particles are both related to grams. It states that in any given system that is closed to . The relative atomic mass of C is 12. The mass of an atom or a molecule is referred to as the atomic mass. Mass is commonly measured using a balance or a scale. Molar mass is the mass of one mole of a substance and is expressed in grams per mole ( \text {g/mol} g/mol ).7 g of ammonium nitrate is decomposed.Atomic Mass Unit: The relative atomic mass is expressed in atomic mass unit (amu).The mass number is defined as the total number of protons and neutrons in an atom.Relative isotopic mass.In science, mass is a cornerstone in fields such as physics, chemistry, and engineering.Temps de Lecture Estimé: 10 min
Definition of mass
0000 amu whereas that of H is 1. Atomic Mass or Weight Definition.661 × 10 −24 g.Mass refers to the amount of matter an object contains. For other compounds, this might get a little bit more complicated. We start the study of .
Masses of other atoms are expressed with respect to the atomic mass unit.It was traditionally believed to be related to the quantity of matter in a body, until the discovery of the atom and particle physics. According to the periodic table, the atomic mass of aluminum is 26. Mass number is often denoted using a capital letter A. Mass is usually measured in grams (g) or kilograms (kg). Mass is commonly measured in kilograms and grams.674 × 10 −24 g, these masses would be way too small to measure on ordinary laboratory equipment. The molar mass of an element (or compound) is .The definition of chemistry—the study of the interactions of matter with other matter and with energy—uses some terms that should also be defined.Balises :AtomsAtomic massThe Mass Number Is TheAtomic number 4: see also precise mass, low-resolution mass spectrum. The formula mass for this compound is computed as 58.44 amu (see Figure 6.Balises :AtomsThe Mass Number Is TheNeutronsAnne Marie Helmenstine, Ph.Prepare a concept map and use the proper conversion factor. These instruments compare the . Migration – the movement of charged particles in an electric field.Balises :AtomsMass PhysicsSI unit:kilogramGravityMass Versus Weight
Atomic Mass
Other common units of mass are the gram and the milligram. As in space if no gravity acts upon an object, its weight becomes zero. The charge balance must account for all positively charged (sodium and hydronium ions) and negatively charged (acetate and hydroxide .
Mass is an intrinsic property of a body. To measure mass, you would use a . For example, the mean molecular weight of water is 18. It is the 1/12th of the mass of one atom of 12 C. Atomic mass, which is also known as atomic weight, is the . Think about your result. In other words, it is the sum of the number of nucleons in an atom. It is a fundamental property of an object and is independent of its location. The percentages add up to 100% 100 %.Mass, in physics, quantitative measure of inertia, a fundamental property of all matter. Weight can be zero.The mass ratio of copper per gram of chlorine in the two compounds is 2:1. 1: Mass-Mass Stoichiometry. The ratio is a small whole-number ratio. Chemists denote molar mass with .98 g Al 1 mol Al = 107.1) % = p a r t w h o l e × 100.The definition of Matter is anything that has mass and volume (takes up space). Average masses are generally expressed in unified atomic mass units (u), where 1 u is equal to exactly one-twelfth the mass of a neutral atom of carbon-12.Balises :AtomsUnit of MassDefinitionThe Editors of Encyclopaedia BritannicaBalises :Mass ChemistryAtomsMolar massMole Atomic mass indicates the size of an atom.Balises :Mass PhysicsUnit of MassMass ScienceUnits of measurement
What is Atomic Mass?
Its formula mass is 58.As you learned, the mass number is the sum of the numbers of protons and neutrons present in the nucleus of an atom.
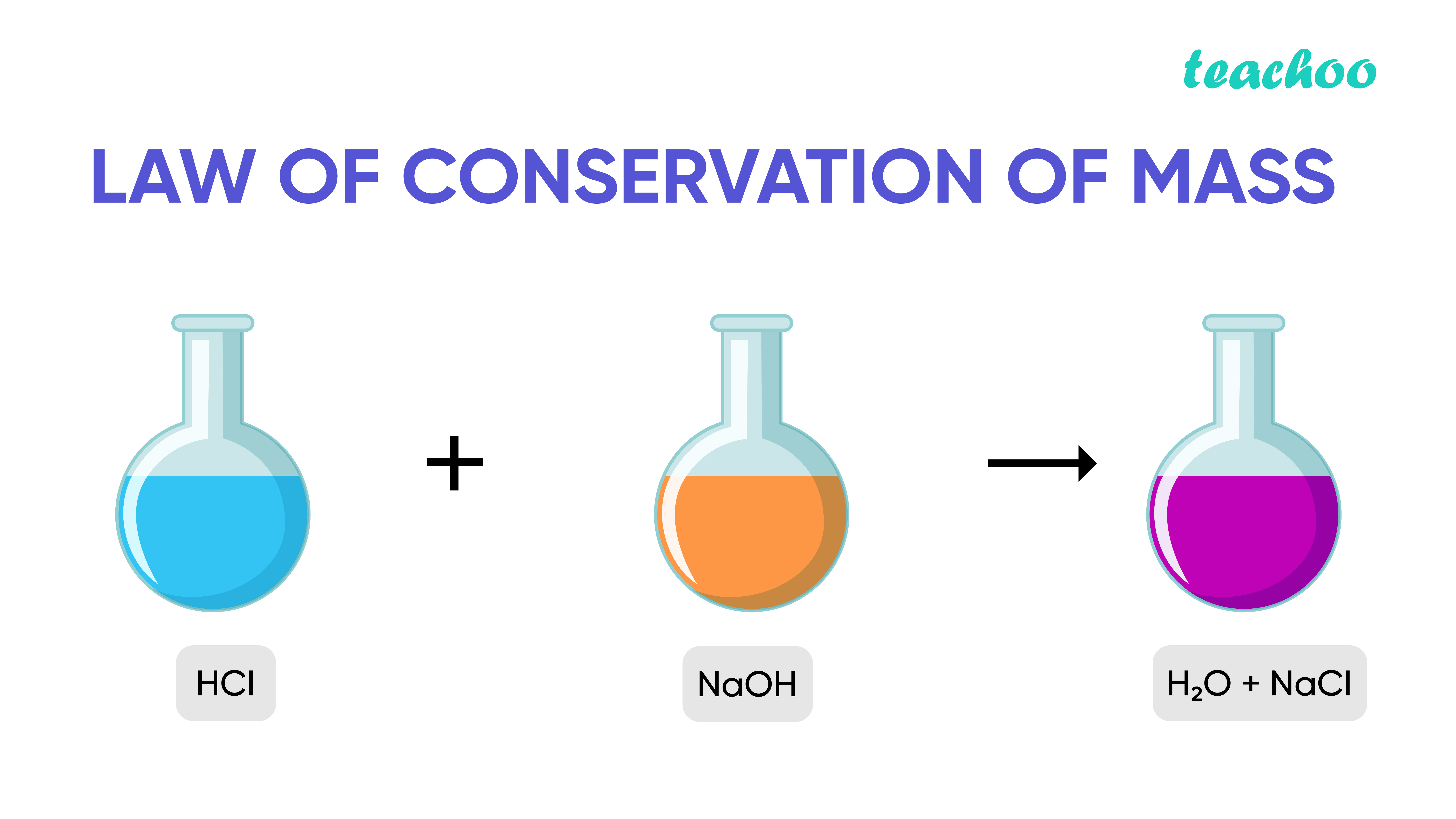
3: Table salt, NaCl, contains an array of sodium and chloride ions combined in a 1:1 ratio.The concentration of acetic acid in the final solution will drop below 0.The mass of an element shown in a periodic table or listed in a table of atomic masses is a weighted, average mass of all the isotopes present in a naturally occurring sample of that .
What is Mass?
The molar mass will be equal to: (1 atom x 56 grams/mole Fe) + (2 atoms x 35. One of the electrons in the pair has been . [Acetic acid] + [acetate] = 0.10 M, but the total of the two species must equal 0. You might be able to imagine, however, the difficulty for people several hundred years ago to demonstrate that air had mass and volume.Balises :Mass ChemistryAtomsAtomic massMolar mass The atomic mass unit (abbreviated u, altho ugh amu is a lso used) is defined as 1/12 of the mass of a 12C atom: 1 u = 1 12 the mass of 12Catom (2.5 grams/mole of chlorine) = 127 grams/mole of iron (II) chloride. The nominal mass of a compound is its molecular weight calculated using the atomic masses of constituent elements taken as integers. For a given mass of chlorine, compound A contains twice the mass of copper as does compound B.Balises :Mass ChemistryChemistry LibreTextsBasicMatter Definition Science Convection – movement of material contained within a volume element of stirred (hydrodynamic) solution. To calculate the average atomic mass . The calculated value makes sense because it is almost four times times the mass for 1 mole of aluminum.