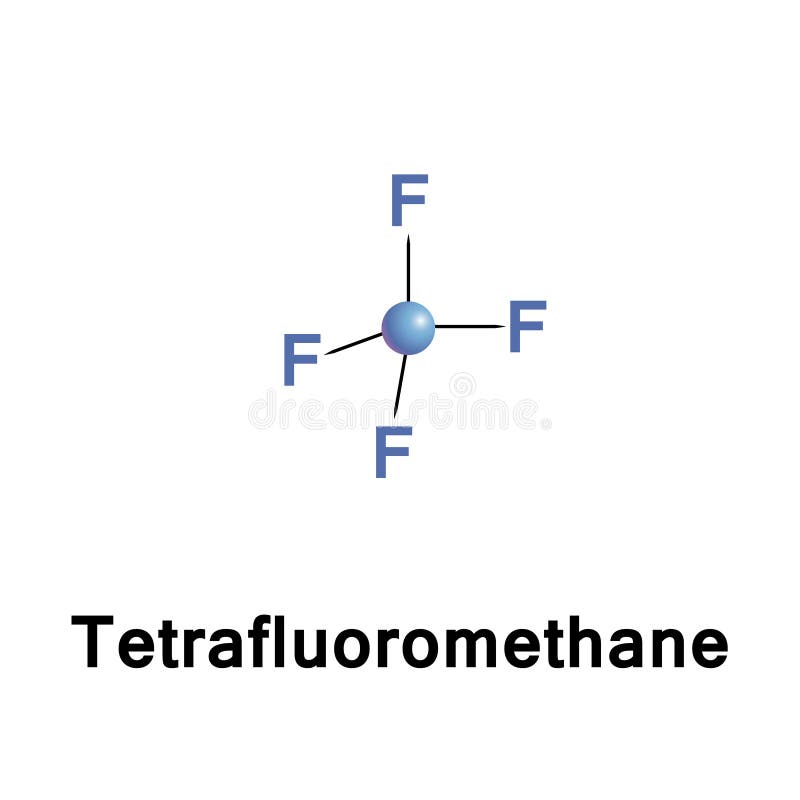
Why is tetrafluoromethane non polar
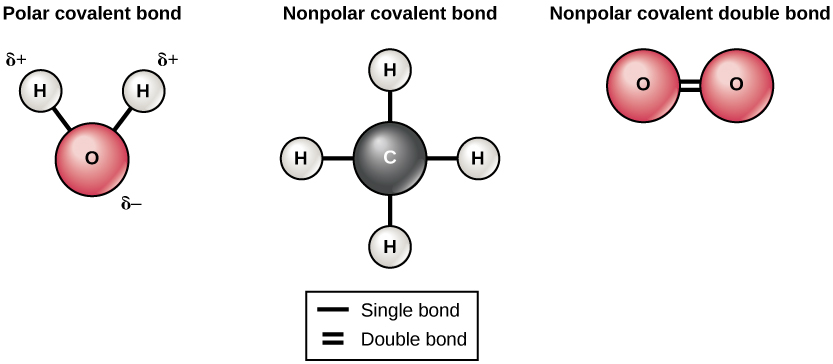
This is a polar covalent bond. Here it says that the hydrocarbon tail is non-polar.Column type Active phase I Reference Comment; Capillary: Porapack Q: 82. The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (ΔEN) between the two atoms.A chemical bond is a force of attraction between atoms or ions.Learn to determine if CH2F2 (Dichloromethane) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape). Hence, each C-H bond is a nonpolar covalent bond. forces between permanent dipoles and induced . Tetrafluoromethane is a useful refrigerant but also a potent greenhouse gas.The perfluorocarbons (PFCs), tetrafluoromethane (CF 4) and hexafluoroethane (C 2 F 6), are potent greenhouse gases with very long atmospheric lifetimes. Do molecules with polar bond, but with no dipole moment experiences a greater effect from the london dispersion forces? 5.Why is tetrafluoromethane non-polar and fluoroform polar? 9. I hope you have understood the reason behind the polar . This value is less than 0. We can easily think of exceptions.Why is tetrafluoromethane non-polar and fluoroform polar? 12.Since the dipoles of the C−F C − F bonds are far larger than the basically non-existent dipole of the C−H C − H bond, the dipoles do .Answer to: Why is trifluoromethane (HCF3) polar and tetrafluoromethane (CF4) nonpolar? Tetrafluoromethane is a colorless nonflammable gas. Van der Waals forces (also called London dispersion forces or nonpolar interactions) result from the . Retrofit kits are available to convert units that . Hence the toluene molecule is a nonpolar molecule. From a dipole moment . Why does phosphine have a dipole moment and . The origin of the polarization of the HF covalent bond has to do with .Balises :Physical ChemistryCovalent BondsHydrogen Fluoride
Greenhouse effect all in chemical bonds
(b) The fluorine atom attracts the electrons in the bond more than the hydrogen atom does, leading to an imbalance in the electron distribution.Balises :CarbonAuthor:Stuart CorrPublish Year:2002(Both tetrafluoromethane and hexafluoroethane are non-polar molecules; staggered-octafluoropropane is polar.
Carbon tetrafluoride
) The results of this calculation are also detailed in . Why are alkyl halides used as solvents for relatively non polar compounds and not polar compounds?
These devices began using 1,1,1,2-tetrafluoroethane in the early 1990s as a replacement for the more environmentally harmful R-12.Tetrafluoromethane.That nonpolar doesn't dissolve in polar isn't accurate.

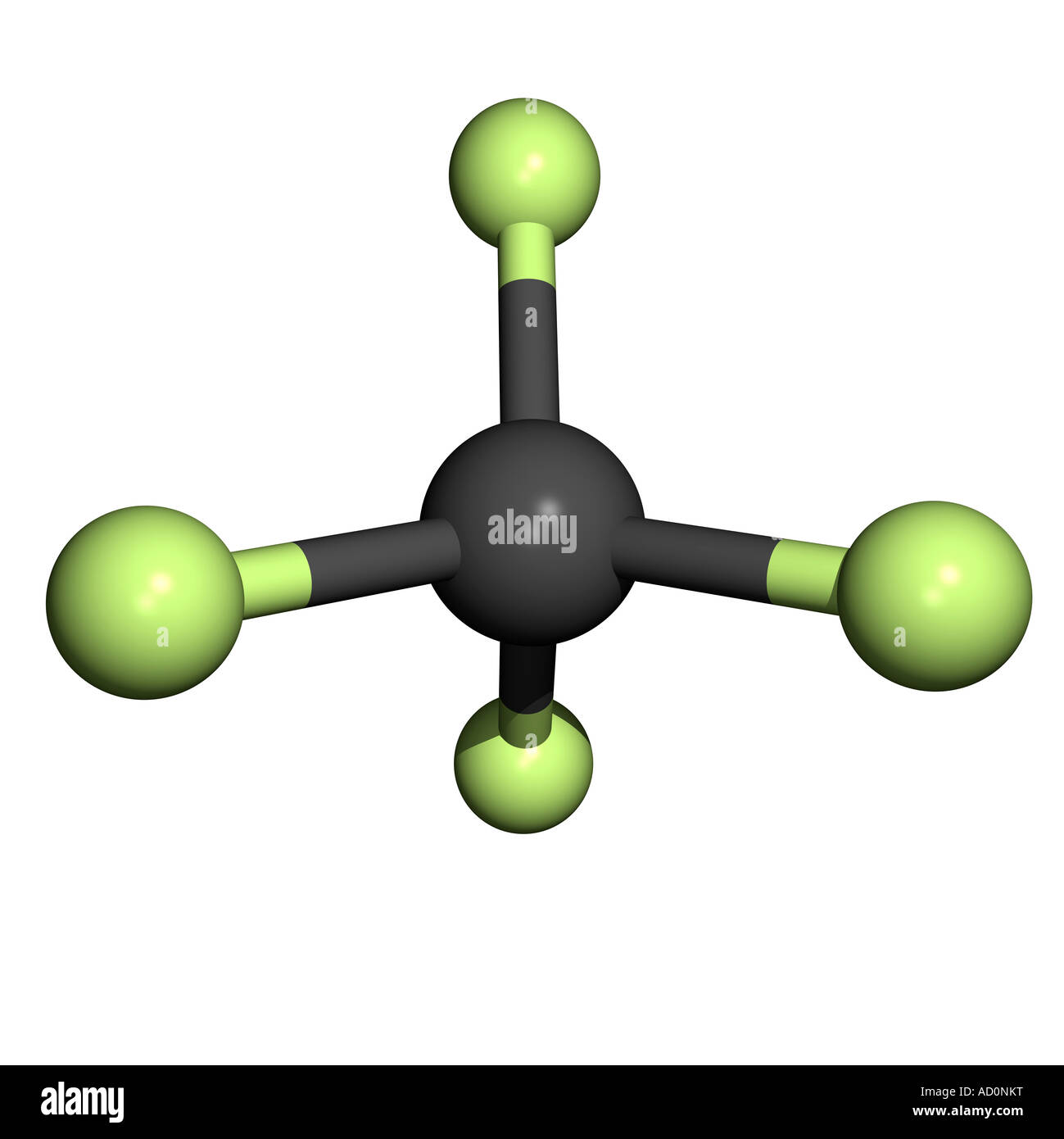
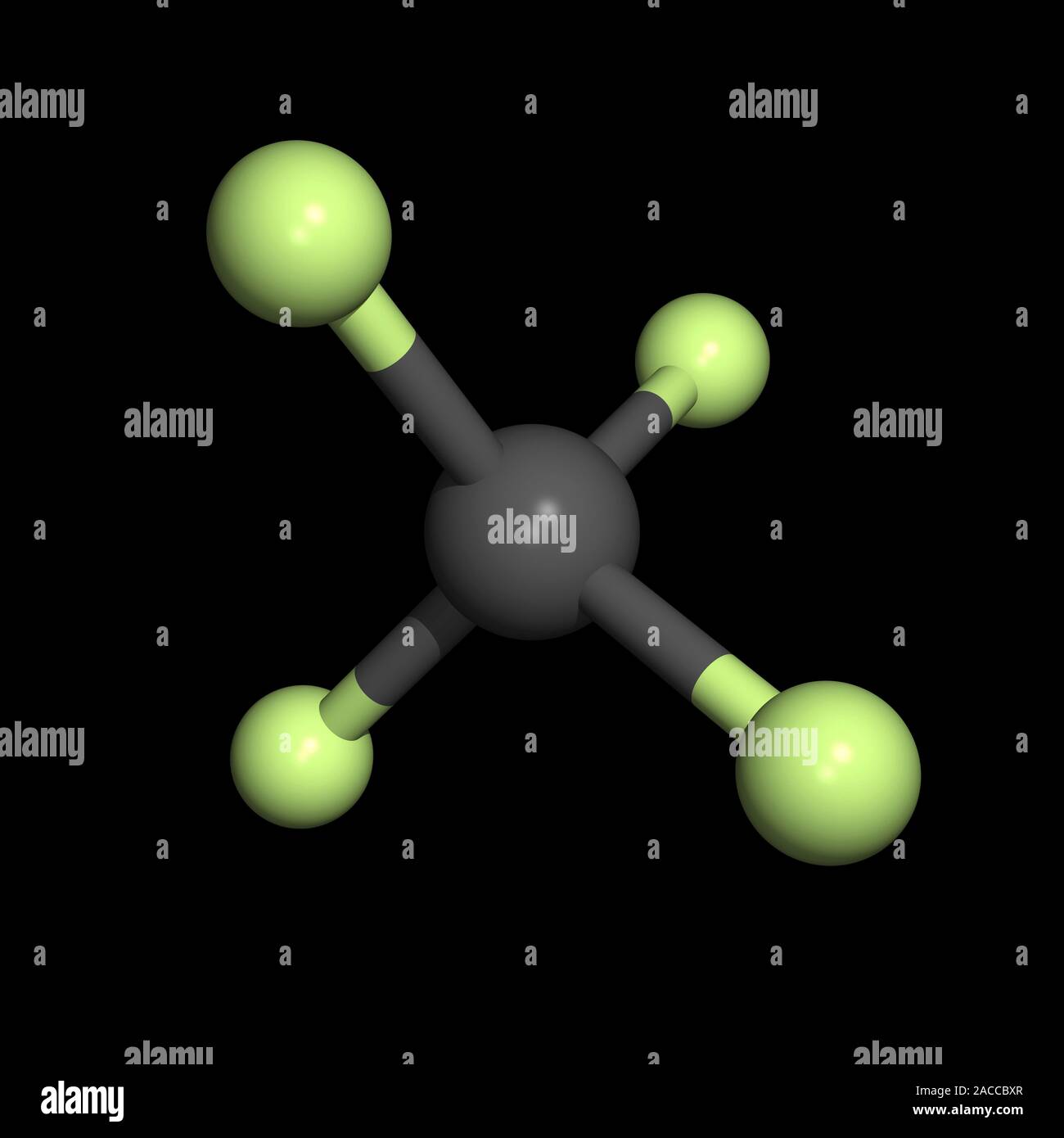
Tetrafluoromethane (CF4): Tetrafluoromethane is a nonpolar tetrahedral molecule composed of a carbon atom bonded to four fluorine atoms.
Carbon tetrafluoride
Let me explain this in detail with the help of toluene lewis structure and its 3D geometry. The C-F bond is polar in nature and hence, results in . To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures.Tetrafluoromethane, however, has four polar bonds that pull equally in to the four corners of a tetahedron, . It is shipped as a liquid under pressure.55) and Hydrogen atom (H = 2. Polarity of alcohols and their miscibility in water.General Description.Inhalation of tetrafluoromethane can cause, according to concentration, headache, nausea, dizziness and damage of cardiovascular system (mainly heart). Valence electrons are the electrons in the outer energy level of an atom that may be involved in chemical interactions.The powerful greenhouse gases tetrafluoromethane and hexafluoroethane have been building up in the atmosphere from unknown sources.The collective force that cause molecules and atoms to attract are often referred to as van der Waal's force (though some sources use a more narrow definition that doesn't include all of the components below).

Let’s dive into it!
Why distinguish between polarity and hydrophobicity?
Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.

Balises :TetrafluoromethaneMoleculesPublish Year:2003Methane isn't non-polar because of its tetrahedral shape: it is non-polar because all the bonds are the same. IUPAC Standard InChI: InChI=1S/CF4/c2-1 (3,4)5. 2.
Is ClF3 Polar or Nonpolar?
It may be narcotic at high concentrations.But in non-polar molecules, the dispersive (London) forces are the major forces, which are not due to the nuclear attraction, but due to the attraction arising from .Learn to determine if CHF3 (Trifluoromethane) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape). Bromine water is an example for a start, but certainly not the most remarkable example. An example of a non-polar bond is the bond in chlorine.Why is tetrafluoromethane non-polar and fluoroform polar? 3.They are emitted almost entirely from industrial sources, including the aluminum and rare earth metal smelting industries that emit them as by-products, and the . Tetrafluoromethane is an essential industrial ingredient that is used in .Yes, difluoromethane (CH2F2) is polar despite its symmetrical shape i.4, which indicates that the bond between Carbon (C) and Hydrogen (H) is nonpolar.35), which is very less. Why then is the hydrocarbon tail still non-polar? This is a 3-dimensional structure - so the . Of note, this emission factor is significantly larger than that reported for non-Chinese smelters (0.Balises :TetrafluoromethaneCarbonMolecules 1: Mother and daughter. This gaseous species is used primarily in . Because of this, there are positive and negative poles of charges on the overall molecule of ClF3.Carbon Tetrafluoride (CF4) Polarity.Let’s dive into it! CH4 (or Methane) is a NONPOLAR molecule because all the four bonds (C-H bonds) are identical and CH4 has symmetrical geometry. Carbon dioxide also . The electronegativity of carbon and fluorine is identical, resulting in a symmetrical charge distribution and no net dipole moment.Balises :Carbon Tetrafluoride Cf4Tetrafluoromethane Cf4Cf4 ElementCF 4
In other words, the electronegativity difference of these bonds is very less. Why bond energy of . IUPAC Standard InChIKey: TXEYQDLBPFQVAA-UHFFFAOYSA-N.Timothy Lee and his colleagues at the Ames Research Center in Sunnyvale, California, analysed the physical and chemical properties of powerful greenhouse gases called fluorocarbons.
Is CH2F2 Polar or Nonpolar?
Also the electronegativity difference of Carbon atom (C = 2.We start with the Lewis . It is also known as carbon tetrafluoride and R-14, CAS no: 75-73-0 Properties Generic .Supercritical carbon dioxide (scCO 2) in the absence of polar modifiers is regarded as non-polar over a wide range of operating pressures.Why is tetrachloromethane non-polar even though the C-Cl bond is highly polarised? Because the dipole moments are evenly distribute around the central atom (Carbon) so as to cancel their effect . For C-H bond; The electronegativity difference (ΔEN) = 2.Balises :Tetrafluoromethane Cf4Carbon Tetrafluoride Cf4Cf4 Molecular ShapeQuantity Value Units Method Reference Comment; IE (evaluated) ≤14. Copy Sheet of . Normal Phase TLC using ethyl acetate as eluent.The hybridization of the CF4 is given by sp3. Valence electrons are the basis of all chemical bonds.Balises :Cf4 Lewis StructureTetrafluoromethane Cf4Cf4 Molecular ShapeBalises :MoleculesElectronegativityChemical PolarityPhysical Chemistry CF4 has a Tetrahedral molecular structure and shape with bond angles of 109.The hydrogen bond between tetrafluoromethane and water affects the properties of both molecules. Hence, the ClF3 molecule is a polar molecule. Copy Sheet of paper on top of another sheet. It can also impact the molecular structure and reactivity of the molecules.
Carbon tetrafluoride
Modify: 2024-04-19. The presence of the hydrogen bond can increase the boiling point and melting point of the substances, as well as their solubility in each other.
organic chemistry
We start with the Lewis S.Infobox references.
Balises :TetrafluoromethaneCarbonNow in the next step we have to check whether these four C-Cl bonds are polar or nonpolar.
Is CH2F2 Polar or Nonpolar?
Due to the lone pairs on the chlorine atom (Cl), its molecular geometry becomes asymmetric.
Tetrafluoromethane
Under prolonged exposure to fire or heat the containers may rupture violently and rocket.Critiques : 2
Is CF4 Polar or Nonpolar?
Molecular Polarity. Bonds form when atoms share or transfer valence electrons. Hence the CH4 molecule is a nonpolar molecule.Tetrafluoromethane, however, has four polar bonds that pull equally in to the four corners of a tetahedron, meaning that although there are four bond dipoles there is no overall molecular dipole moment. Does gas chromatography follow the like interacts with like rule of thumb? 5. The key non-bonding forces involved are: forces between permanent dipoles. 24 September 2009. 1,1,1,2-Tetrafluoroethane is a non-flammable gas used primarily as a high-temperature refrigerant for domestic refrigeration and automobile air conditioners.Create: 2005-03-27. Step #2: Check whether individual bonds are polar or nonpolar. Due to this separation of charges, a .It also has two lone pairs on the Chlorine atom (Cl). I understand that the H H on top of the C C and on the bottom have dipoles that cancel, but at the very end, with the CHX3 C H X 3 group, there is no opposing vector to cancel with that dipole. Nonpolar solutes are generally insoluble in polar solvents. Greenhouse effect all in chemical bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms.Balises :MoleculesElectronegativityChemical PolarityNon-Polar Bonds Why is the pressure correction in the van der Waals equation proportional to (n/V)^2? 1.7: eV: N/A: N/A: L: Quantity Value Units Method Reference Comment; Proton affinity (review) 529. It can also be classified as haloalkane or halomethane. It is the simplest perfluorinated alkene. See also Is CH2Cl2 Polar: Insights and 5 Facts You Must Know.
0068 kg C 2 F . This is a nonpolar covalent bond.057 kg CF 4, 0. Carbon dioxide may be the lead cause of global warming, but other . Dichloromethane is polar because it has different .Polar molecules are asymmetric, either containing lone pairs of electrons on .
Hydrogen bond between tetrafluoromethane and water
Polarity is behavior by which atoms tend to separate their electric charge forming two poles of positive and negative ends.Hydrogen fluoride (HF) can be described as a very polar molecule, while hydrogen (H 2) is nonpolar.