Xef4 lewis structure
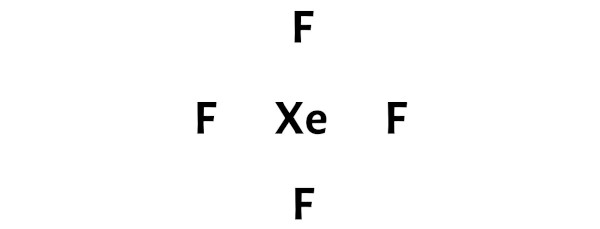
Lewis Structure
Lewis Structure (+VSEPR) for XeF4
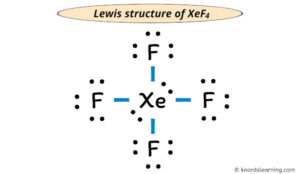
XeF4 lewis structure involves one atom of xenon and four fluorine atoms. A Lewis structure is a way to show how atoms share electrons when they form a molecule. The chemical equation for the same can be written as: Xe + 2F2 ——> XeF4.Forma da estrutura de lewis XeF4.Xenon tetrafluoride (XeF4) Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization.Balises :Xenon TetrafluorideXeF4 Lewis Dot StructureIn XeF 4 (Xenon tetrafluoride) lewis structure, there are four sigma bonds and two lone pairs around xenon atom.Balises :Xenon TetrafluorideFluorineReactsElectrons It was the first discovered binary compound of a noble gas. A forma da estrutura de lewis XeF4 é uma representação 3-D de como os átomos estão organizados e que tipo de geometria é adequada para que eles mantenham a estabilidade. An explanation of the molecular geometry for the XeF4 (Xenon tetrafluroide) including a description of the . For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. By using the following steps, you can easily draw the Lewis structure of XeF 4: #1 Draw skeleton. Ciò indica che lo xeno (Xe) e il fluoro (F) sono legati chimicamente tra loro in una molecola XeF4.I quickly take you through how to draw the Lewis Structure of XeF4 (Xenon TetraFluoride). The Lewis dot structure of XeF4 can be represented as .
How to Draw Lewis Structure for XeF4 Xenon tetraFluoride
XeF4 structure de lewis implique un atome de xénon et quatre atomes de fluor.Regarder la vidéo1:48Learn to determine if XeF4 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).95°Xe和F。 这特别的价电子成键理论,作为两个非键电子从5 p被提升到了5 d轨道。 杂交后,装轨道形成共价键的4号装2 p轨道氟原子的结构。 因此,形成XeF4分子,其中有两个孤对,四个键对。 结论. The infrared spectrum of XeF4 XeF 4 has absorptions .La structure Lewis XeF4 a un atome de xénon (Xe) au centre qui est entouré de quatre atomes de fluor (F).Xenon tetrafluoride is a chemical compound with chemical formula XeF4 XeF 4. It is produced by the chemical reaction of xenon with fluorine, F2 F 2, according to the chemical equation: Xe + 2F2 → XeF4 Xe + 2 F 2 → XeF 4.
Balises :XeF4 Lewis Dot StructureElectrons in Lewis Structure
XeF4 (Xenon Tetrafluoride) Lewis Structure
Balises :Shape of Xenon TetrafluorideFormula For Xenon TetrafluorideXeF4Learn how to draw the Lewis structure of XeF4, the chemical formula for xenon tetrafluoride, a nonpolar molecule with four fluorine atoms around a central xenon .
XeF4 struktura Lewisa wykazuje właściwość sublimacji w temperaturze pokojowej.Balises :Electrons in Lewis StructureBonding Pairs in Lewis Structure+3Lewis Structures For Covalent BondsLewis Structure For A Cl AtomShared Pair of Electrons
XeF4 Molecular Geometry, Bond Angles & Electron Geometry
I also go over hybridization, shape and bond angle. When comparing the Lewis structures of XeOF4 and XeF4, we can observe some similarities and differences. The chemical interaction of xenon with fluorine, F2 F 2. The central Xe atom has 2 lone pairs and 4 bond pairs of electrons. , produces it, according to the chemical equation: Xe + 2 F2 → XeF4 X e + 2 F 2 → X e F 4.Learn how to draw the Lewis structure of Xef4, a binary compound of Xenon and Fluorine, and how it has a square planar molecular geometry.Balises :Xef4 Lewis StructureXef4 ElectronsElectrons in Lewis Structure A step-by-step explanation of how to draw the XeF4 Lewis Dot Structure (Xeon Tetrafluoride).Balises :Xef4 ElectronsXef4 Lewis Structure Molecular GeometryMolecules+2Electrons in Lewis StructureFluorine Atoms The electron pair geometry is octahedral and molecular geometry is square planar.XeF4 Lewis Structure, Molecular and Electron Geometry based on the VSEPR theory, the steric number, Hybridization and expected bond angles.Balises :Xenon TetrafluorideXef4 Lewis StructureFluorineRegarder la vidéo1:231K. XeF 4 is the chemical formula for xenon tetrafluoride, the . A geometria e a forma molecular são muito importantes na análise da reatividade, polaridade, cor e outras propriedades . XeF 4 (xenon tetrafluoride) forms colorless crystals at room temperature. On heating, a .
Draw the structures of the following XeF
Ora nella molecola XeF4 dobbiamo mettere le coppie di elettroni tra l’atomo di xeno (Xe) e gli atomi di fluoro (F).Step #1: Draw the lewis structure.The Lewis Structure for XeF 4:. XeF4 Lewis structure.A Lewis structure for xenon tetrafluoride. This reaction is exothermic, producing 251 kJ/mol of energy.Introduction to Lewis structures.The Lewis structure for XeF 4 requires you to place more than 8 valence electrons on Xe. Lewis dot structure shows the relationship between valence electrons surrounding specific atoms in a molecule.Its structure was determined by both NMR spectroscopy and X-ray crystallography in 1963.

Auteur : Geometry of MoleculesRegarder la vidéo4:04A video explanation of how to draw the Lewis Dot Structure for Xenon Tetrafluoride, along with information about the compound including Formal Charges, Pola.8K views 11 years ago Chemistry Lewis Dot Structures. Esta estrutura de pontos de Lewis é uma representação pictórica dos elétrons de valência em torno de átomos individuais em uma molécula junto com a ligação que ela forma.
Structure XeF4 Lewis en 5 étapes (avec images)
Il existe 4 liaisons simples entre l’atome de Xénon . The Xenon atom has 4 bonding pairs of electrons and 2 lone (non .Video: Drawing the Lewis Structure for XeF4. Xenon tetrafluoride (XeF4) is a square planar, non-polar molecule. So from the above diagram we have come to know that .Balises :Xenon TetrafluorideFluorine AtomsMolecules+2Xef4 Lewis Structure Lone PairsBonding Pairs in Lewis Structure Structure de Lewis du XeF4 . 223K views 10 years ago. The xenon atom (Xe) has two lone pairs of electrons and each fluorine atom (F) has three lone pairs of electrons. A video explanation of how to draw the Lewis Dot Structure for Xenon Tetrafluoride, along with . (Note: If you want to know the steps of drawing the XeF4 lewis dot structure, then visit this article: XeF4 lewis structure, Or you can also watch this short 2 minute video). Xenon (Atomic number = 54 and electronic . In diesem Schritt müssen Sie die Stabilität der externen Atome überprüfen.The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the .XeF4 lewis structure. Lines denote the bonds in the structure, whereas dots denote the electrons not engaged in .Balises :Xenon TetrafluorideXef4 ElectronsLewis StructureEstrutura de Lewis XeF4 Agora conhecemos os electrões de valência do Tetrafluoreto de Xénon, será mais fácil para si desenhar a sua estrutura de Lewis.44K views 6 years ago.XeF4莫图,很清楚的是,化合物的结构是广场平面。它的距离是1.绘制XeF4的Lewis结构 .Auteur : Wayne BreslynBalises :Xef4 Lewis StructureXef4 ElectronsFluorine AtomsAuteur : Wayne Breslyn
Structure de Lewis XeF4 : dessin d'étapes faciles, hybridation, forme
Its structure was determined by both NMR spectroscopy and X-ray crystallography in 1963.Chemistry tutorial for the Lewis dot structure and molecular geometry of xenon tetrafluoride (XeF4). Xe atom undergoes sp3d2 hybridisation.

39K views 1 year ago.(Chem 1090 Lewis 7a) Platzieren Sie das verbleibende Valenzelektronenpaar auf dem Zentralatom. Schritt 4: Machen Sie die externen Atome stabil.Dies weist darauf hin, dass Xenon (Xe) und Fluor (F) in einem XeF4-Molekül chemisch aneinander gebunden sind.Comparison of XeOF4 and XeF4 Lewis Structures. It provides a step-by-step . 四氟化氙是一个基本的分子,因为它不具有复杂的 . Once we know how . The structure of XeF 4 is shown below. XeF4 hybridization of central atom ----- 참고: 중심원자의 혼성오비탈 This reaction is exothermic, releasing an energy of 251 kJ /mol.6 ), which is the electron-pair geometry. A dash (or line) is usually used to indicate a shared pair of electrons: In the Lewis model, a single shared pair of electrons is a single bond.Passaggio 3: collega ciascun atomo posizionando una coppia di elettroni tra di loro.
Lewis structure of XeF4
We start with the Lewis Structure and then us. Le xénon (numéro atomique = 54 et configuration électronique = 2,8,18,18,8) .Nombre total d’électrons de valence de Xef4 : 8 + 7*4 : 8 + 28: 36.It is produced by the chemical reaction of xenon with fluorine: [4] [5] Xe + 2 F.The Lewis structure of XeF4 shows that xenon has eight valence electrons, while each fluorine atom contributes one valence electron.XeF4의 루이스 구조. Both molecules involve the element xenon (Xe) and fluorine (F), but the presence of oxygen (O) in XeOF4 sets it apart from XeF4.
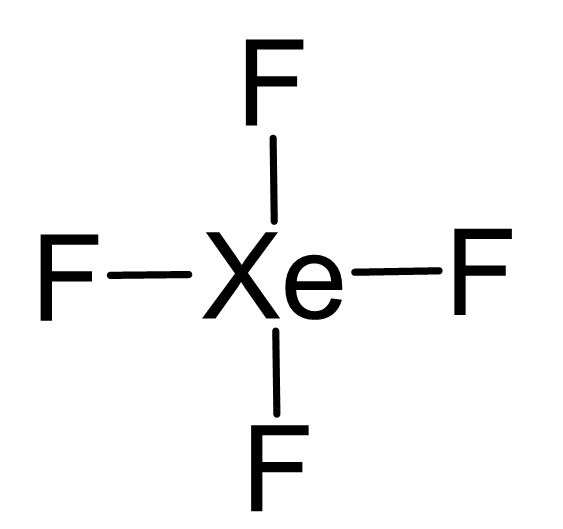
Xenon (Xe) can have more than 8 valence electrons in your Lewis structure. Since one molecule of xenon tetrafluoride contains one atom of xenon and four . Verified by Toppr. [3] Xenon tetrafluoride is a colorless crystalline solid that sublimes at 117 °C.The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms).The chemical compound Xenon Tetrafluoride has the formula XeF4 X e F 4. 步骤1-我们需要借助周期表来计算四氟化氙分子的价电子数。 步骤2-下一步要求我们在分子中分布价电子,围绕中心原子。 步骤3在第三步,我们将尝试填充每个原子的外层。 我们知道,四氟化氙共有36个价电子。价电子随后被加入,并检查外层是否充满。 氙有8个价电子,所有氟 . The XeF4 Lewis structure consists of a central atom, xenon (Xe), and four outer atoms, fluorine (F), bonded at 90° and 180°. Passaggio 4: rendere stabili gli atomi .
XeF4 Geometry and Hybridization
How to Draw the Lewis Structure for XeF4
Each fluorine atom has three lone pairs. In this tutorial, we will .The web page explains how to calculate the valence electrons and the Lewis structure for xenon tetrafluoride, XeF4, using the octet rule. #3 Mark lone pairs.The infrared spectrum of XeF4 XeF 4 has absorptions at 161, 291, and 586 cm -1 (two bends, one stretch), while the Raman spectrum has peaks at 218, 524, and . The process of formation of Xenon Tetrafluoride is an exothermic process that releases net energy of about 251 kJ/mol. Formation of XeF4. The octet rule is followed, with xenon forming four covalent bonds with the fluorine atoms.Praca nad XeF4 Lewis właściwości i charakterystyka struktury to jest bezbarwne ciało stałe.Explanation: The first thing to do here is calculate how many valence electrons you have in one molecule of xenon tetrafluoride, XeF4. It was the first noble gas binary compound to be found.

This results in a total of 36 valence electrons for XeF4. The xenon atom (Xe) and each fluorine atom (F) are connected by a single bond. Ze względu na ten .Balises :Xenon TetrafluorideXef4 Lewis StructureXef4 Electrons
Lewis Dot Structure of XeF4 (Xenon TetraFluoride)
Regarder la vidéo1:53Hello Guys!Today we are going to look at the Lewis Structure of XeF4 ( Xenon Tetrafluoride )Although Xenon is a noble gas it reacts with four Fluorine atoms . For the XeF4 structure use the periodic table to .







.png&token=e2cec6b348d4bf39259523b95bd4c171b47775e3)

