Bioburden and endotoxin relationship
Evidence against a bacterial endotoxin . Corpus ID: 80959183.Graphically, there seemed to be some correlation and possible bioburden prediction value; however, the data did not demonstrate a statistically significant .
Analytical method transfer and processes for bacterial endotoxin testing
Understanding bioburden Potential microbial contaminants can take many forms, including bacteria, viruses, and molds. An understanding of the properties of these various potential contaminants can help us mitigate risks. Traditional endotoxin assays are time-consuming, error-prone, and difficult to perform. A low alert level (product/facility history) and a higher action level, at or below the EMA criterion would be appropriate.
Bioburden and Endotoxin Control in Pharmaceutical Processing | Semantic Scholar.Bioburden testing for terminally sterilized medical devices is performed according to ISO 11737-1. The pre-established bioburden and endotoxin limits should be provided (3. A frequent source of endotoxins and bioburden is water, which can carry Gram negative bacteria. Our 3rd party lab has mentioned $110 for the 4 test-bioburden analysis and about $110 for Endotoxin LAL test.
Annex 1 and Contamination Control Strategies
Difference Between Bioburden and Endotoxin Testing Endotoxin testing determines bacterial endotoxin, a phospholipid found in the outer membrane of gram . For a product to carry any kind of claim, such as “endotoxin free” or “non-pyrogenic”, tests must be carried out to prove this.
Manquant :
bioburdencomRecommandé pour vous en fonction de ce qui est populaire • AvisThe Biologics Revolution and Endotoxin Test Concerns
There should be working limits on contamination immediately before sterilization, which.As nouns the difference between endotoxin and bioburden is that endotoxin is any toxin secreted by a microorganism and released into the surrounding environment only when it dies while bioburden is.Bioburden and endotoxin levels before and after the maximum allowed hold time should be monitored and bioburden and endotoxin limits provided. Bioburden sampling should occur prior to any 0. An outline of bioburden and endotoxin sampling expectations for monoclonal . Pyrogen and Endotoxins Testing: Questions and Answers. – Sterilization and .While bioburden focuses on the total microbial load, endotoxin specifically targets the presence of endotoxin from Gram-negative bacteria.For biologics manufacture there are many bioburden/endotoxin sampling expectations.

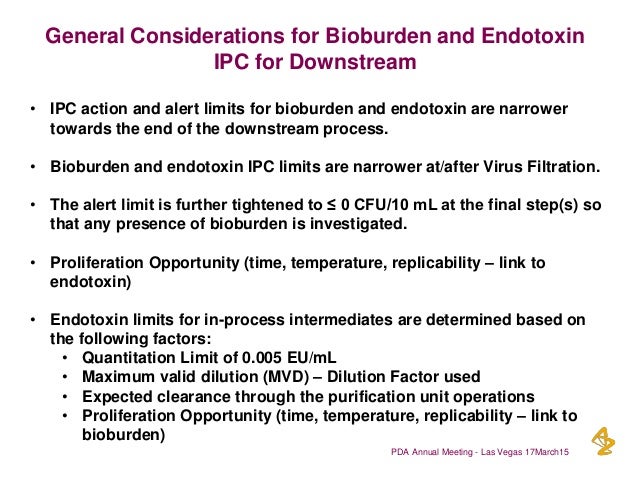
In book: Biocontamination Control for .Though bioburden testing may be referred to synonymously with microbiology testing, microbiology testing allows for the specific type of live microorganism to be identified and quantified. Historical options for BET include traditional 96-well microplate assays that are manual and not ergonomically friendly .Bioburden assessment informs the. • The alert limit is further tightened to ≤ 0 CFU/10 mL at the final step(s) so that any presence of bioburden is investigated.High bioburden acceptance criteria should not be justified by the capacity of the sterilisation process or any bioburden reducing step before sterilisation. Bioburden testing follows the methods outlined in USP 60, USP 61, and USP 62.
Bioburden and Endotoxin (LAL) Testing proposal help
Office of Regulatory Affairs (ORA) June 2012. The levels of bacterial endotoxins in the finished product can be impacted by the bioburden and bacterial endotoxins in the components (i.To evaluate column and UF/DF performance perform bioburden/endotoxin testing on an appropriate number of batches at scale.Remove the bioburden testing bottleneck in product release; More efficient endotoxin testing. Food and Drug .Bioburden and Endotoxin Testing Play a Key Role in The Assessment of Microbial Risk of Non-Sterile Process Intermediates.
There are various ways to test products for pyrogens, specifically endotoxin, and the foremost method remains the Limulus Amebocyte Lysate (LAL) test.Bacterial Endotoxin:Molecular Relationships Between Structure and Activity - ScienceDirect.
Aseptic Processing of Biological Products: Current Regulatory Issues
Bioburden
I have done some initial cost analysis to figure out how much the Bioburden and Endotoxin tests will cost per lot. active substance, excipients and containers), and by microbiological contaminants introduced during manufacture.2 µm filtration step.2, 63-72 *Corresponding author. • Proliferation Opportunity (time, .Assessing Process Hold Times for Microbial Risks: Bioburden and Endotoxin.It reacts specifically with bacterial endotoxin, highlighting its presence. If we are running production 8 times a month requiring 10 samples for bioburden and endotoxin (according to std) we . manufacturer about both the expected microbial load of the product and the presence or. When cutting up and disassembling the test unit, extensive manipulation may be necessary . This paper has discusses the implications of the process hold times on microbial growth . In addition, there are risks of exotoxin or endotoxin .Bioburden suitability (item 1 of the four requirements) is assessed when a product contains antimicrobials or some inhibitory substance/properties that might interfere with recovery . IND 103031 Page 6 U.22 µm membrane during aseptic filling, .Bioburden test quantifies viable or live microorganisms present on a medical device or product.Answers by EMA on the Topic Bioburden - ECA Academy - .

orgWhat is a reasonable Bioburden limit? - Elsmar Cove . Subvisible Particulates Testing Particulates of all sizes are of interest to regulators and producers of pharmaceutical products, and a great deal of industry effort is spent trying to determine particulate distributions per USP 788 .Introduction Control of bacterial endotoxins, gram-negative bacteria that can cause pyrogenicity, is critical in the manufacture of pharmaceutical drug products intended for parenteral administration. It is proposed that any excursion warrants a thorough investigation and a case-by-case assessment for .Bioburden and endotoxin testing traditionally have been performed retrospectively, and only through sufficient validation and historical data is sufficient confidence developed. It has been suggested that endotoxin cannot be detected over time in certain biopharmaceutical dr .Endotoxin Vs Exotoxin - Definitions, Examples and . Online water bioburden analyzers (OWBAs) have the potential to eliminate sampling and testing errors via reduced manipulations, while providing . Tim Sandle, in Biocontamination Control for Pharmaceuticals and Healthcare, 2019.Endotoxin testing allows universal detection of bacterial endotoxins, while direct detection of other toxins and non-endotoxin PAMPs (pathogen-associated molecular .microscopemaster.
Ethide Laboratories
This study therefore demonstrates a quantitative relationship between high endotoxin levels in plasma and the development of septic shock and multiple organ failure.Wednesday March 17, 2021 12:00 pm – 1:30 pm EDT. Guidance for Industry. Reference ID: 4754307 .
Monitoring of Bioburden
Endotoxin testing using the compendial LAL .
Comparison of bacterial and endotoxin retention by charge
As per the regulations, The bioburden should be monitored before sterilization.
Bioburden Testing: Purpose, Procedure, and Accepted Level
Pyrogenicity and bioburden testing ensure that cosmetic products are free of unwanted microbes and pyrogens so that users will not be at risk of illness following product use. Resin storage conditions should be tested for bioburden. Whereas bacterial endotoxin test detects, and estimates endotoxin .Acceptance criteria for bioburden are discussed under the relevant sub-sections of 4. [1] The term is most often used in the context of bioburden testing, also . Biocontrol Science, 2016, Vol.“Bioburden is a potential risk to the patient not only because the sterilization process might not be completely effective, but also postprocessing because of the . It is therefore important to examine . Microbial testing performed in support of pharmaceutical and biopharmaceutical production falls into three main categories: detection (qualitative), . In addition, there are risks of exotoxin or endotoxin contamination. The author presents some of the rapid method technologies under evaluation or in use by pharmaceutical microbiologists and the current status of implementation of alternative microbial methods. • Bioburden and endotoxin IPC limits are narrower at/after Virus Filtration.
What is the Difference Between Bioburden and Endotoxin Test?
Microbial testing performed in support of pharmaceutical and biopharmaceutical production falls into three .Bacterial endotoxin is a Gram-negative bacterial cell wall component that is harmful to humans at threshold concentrations, and it is not expected to be in aseptically-produced pharmaceutical medicines.Endotoxin/bioburden test protein pool (filtered or unfiltered) at the end of chromatography and UF/DF operations. absence of specific microorganisms, . Enter two words to compare and contrast their definitions, origins, and synonyms to better understand how those . Overall, testing for pyrogens, particularly bacterial endotoxins, and bioburden are imperative safety measures for regulatory approval of cosmetic products.
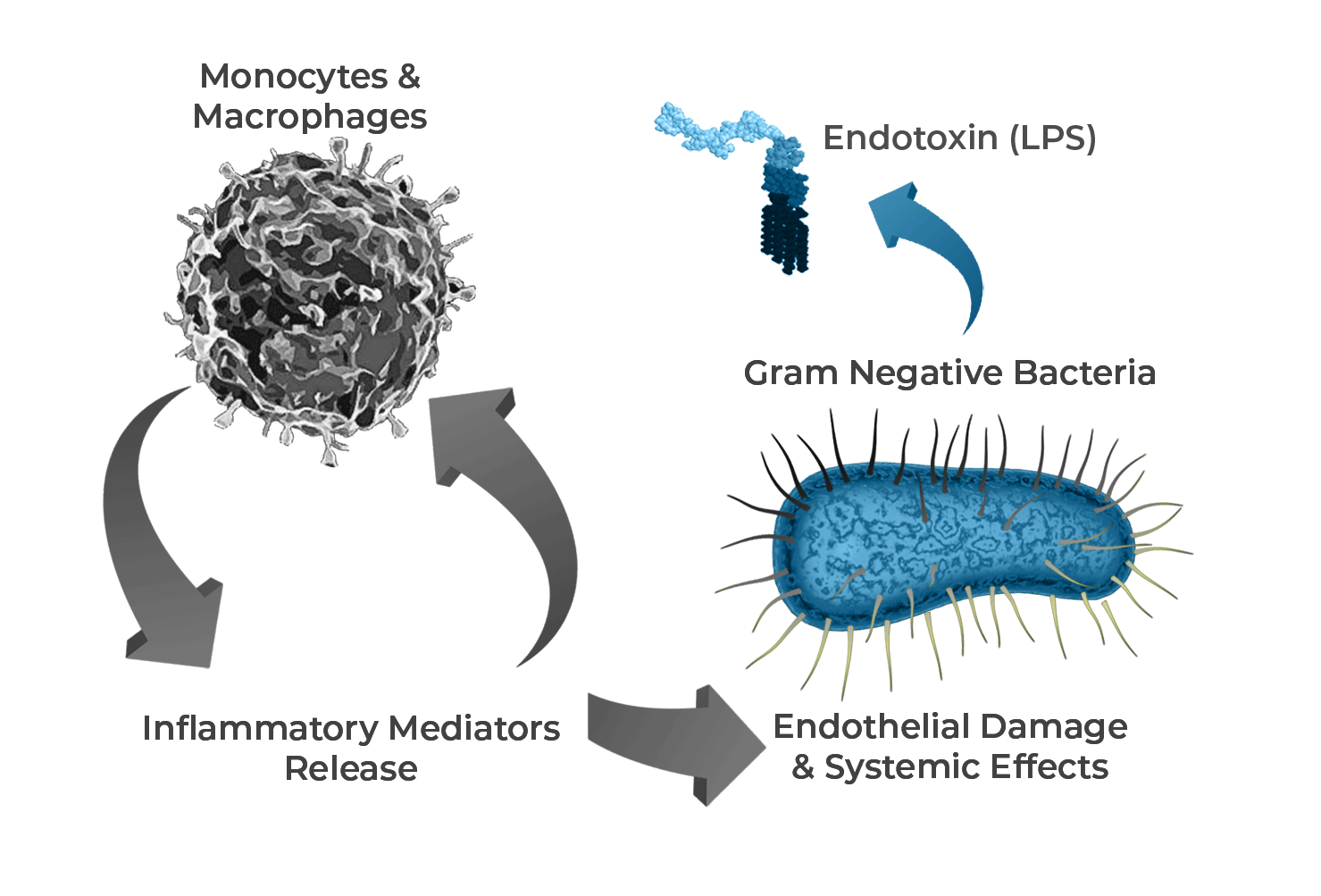
What's the difference between and .Bacterial endotoxins cause a pyrogenic response when injected into the human bloodstream at sufficient concentration. • Proliferation Opportunity (time, temperature, replicability – link to endotoxin) • Endotoxin limits for in-process intermediates are determined based . Bioburden and Endotoxin . This chapter has looked at bioburden and endotoxin testing during pharmaceutical processing.Bioburden is normally defined as the number of bacteria living on a surface that has not been sterilized.Bioburden quantifies viable microorganisms because microorganisms are of infectious concern while alive.

On the other hand, in cold water systems [WFI and deionized (DI)], both bioburden and endotoxin control are .gmp-compliance. Bioburden testing is primarily performed by cutting up, disassembling, or flushing the fluid path of the test unit using sterile tools to prepare the sample.Key words: Rapid Assay / Bioburden / Endotoxin / Contamination. This knowledge hascomRecommandé pour vous en fonction de ce qui est populaire • Avis Take bioburden and endotoxin samples from the final bulk drug substance (post filtration). The microbial detection control strategy of large scale biologics production defines bioburden and endotoxin sampling points for nonsterile process intermediates. Innovative technologies that test for these parameters align with the guidelines from Annex 1 to enable better process . Additional copies are available from: Office of . LAL testing is an established part of many quality control (QC .comComparing Endotoxin Detection Methods - PharmTechpharmtech. Volume 5, Issue 4, .A holistic approach to bioburden risk is recommended, using a two-tier system for low bioburden monitoring prior to sterilization.• IPC action and alert limits for bioburden and endotoxin are narrower towards the end of the downstream process.
Ethide Laboratories
1016/B978-0-12-814911-9. Here is a brief overview of how it’s performed: Sample Collection.How is bioburden testing performed? The goal of bioburden testing is to quantify and identify microorganisms to ensure products meet specified quality standards and regulations. Bioburden assessment informs the manufacturer about both the expected microbial load of the product and the presence .