Breast implants recalled

The company has also filed a motion to honor its return and refund policy, benefiting surgeons who may have unused breast implants on hand which they wish to return.
However, officials estimate this represents only about half (55%) of the number of cases . Thousands of women are missing from the breast implant register set up as a result of the PIP breast implant scandal, new figures suggest.
If you have inventory of the recalled products, Quarantine product to prevent its use. Textured breast implants made by Allergan that have been linked to an unusual cancer are being recalled in the United States at the request of the Food and .Support provided by Allergan, Mentor and Sientra.Tue 9 Nov 2021 14.
Mentor Breast Implants: What You Need to Know
In most cases, removing the implant and the capsule .The FDA ordered Allergan to recall some of its textured breast implants and tissue expanders to protect consumers from the risk of developing a lymphoma. More than 580,000 beds recalled because they can break or .

Even if your breast implants are being cancelled, suspended or recalled, medical experts do not recommend removing them if you do not have symptoms of BIA-ALCL.When the patient and physician begin discussing clinical implications of recalled breast implants, the nature of a recall, symptoms and signs, risks and benefits of and alternatives to explantation, limitations of present knowledge, and financial considerations must be carefully considered.comHere’s How That Breast Implant Recall Is Linked to Cancer | .Written by: Stacy Simon Senior Editor, News. However, Allergan smooth and Allergan BRST Microcell breast implants have not been recalled and are still available.) It allocates $600,000 for this program. Some breast implants have been removed from the Australian market. The FDA has not released the exact number of implants affected .Have Mentor breast implants been recalled? Mentor implants have not been recalled due to BIA-ALCL risk.The FDA says the risk of lymphoma with Allergan's Biocell textured implants, the implants that have been recalled, is approximately six times that of other manufacturers who are selling in the U.The recall means all Allergan breast implants of the Biocell macro-textured type from the Natrelle product range have been returned to the supplier and are no longer available.Allergan are working with their notified body GMED, based in France, to resolve the issue. This is a real possibility, especially for those who have received textured breast implants. Update - Suspended breast implant devices now cancelled.A Biocell textured breast implant by Allergan Aesthetics was recalled by the company in May 2019, after the product was linked to breast implant-associated anaplastic large cell lymphoma, a rare .The July 24, 2019 announcement that Allergan voluntarily recalled their BIOCELL textured breast implants at the request of the FDA has spread quickly, leaving many women feeling confused and concerned about how this decision may personally impact their lives.Breast implants are a popular choice for breast augmentation, but not all implants are created equal.The FDA’s warning letter to Allergan noted several serious deficiencies in the manufacturer’s post-approval study to evaluate its NATRELLE Silicone Gel-Filled Breast Implants . 350-2200 350-2225 350-2250 350-2275 350-2300 350-2325 350-2350 350-2375 350-2400 350-2425: SMOOTH ROUND HIGH PROFILE SALINE-FILLED MAMMARY PROSTHESIS: More . The pharmaceutical company Allergan has recalled all BioCell textured breast implants at the request of the US Food and Drug Administration (FDA) because they have been linked to a rare type of .Textured breast implants made by Allergan were recalled globally this week after the US Food and Drug Administration reported an increased number of cancer cases and deaths linked to the products . This is because BIA-ALCL is very rare, and the risk of undergoing surgery could be higher than the risk of developing BIA-ALCL.comRecommandé pour vous en fonction de ce qui est populaire • Avis
What to Know About Breast Implants
Textured breast implants made by Allergan that have been linked to an unusual cancer are being recalled in the United States at the request of the Food and Drug Administration, and .FDA News Release: FDA takes action to protect patients from risk of certain textured breast implants; requests Allergan voluntarily recall certain breast implants and tissue .How do we find out if our implants were part of the recall .The French government immediately recalled the implants.Consumer Updates.

Investigations.Breast implant maker Allergan Inc.Allergan is taking this action as a precaution following notification of recently updated global safety information concerning the uncommon incidence of breast implant-associated . Food and Drug Administration (FDA) has requested that pharmaceutical company Allergan recall its BIOCELL textured breast implants due to a .The manufacturer of Biocell breast implants recalled all models of the implant on Wednesday, two months after Health Canada banned them from sale in this country amid concerns over a heightened .When the patient and physician begin discussing clinical implications of recalled breast implants, the nature of a recall, symptoms and signs, risks and benefits of and . Later in June 2012, a UK report found that PIP implants had double the rupture rate of other implants. (None of the company’s devices, including Sientra breast implants, have been recalled. Manufacturer Mentor Texas, LP. These implants have been linked to a rare form of cancer called BIA-ALCL, which can develop around the scar capsule that ., 3025 Skyway Cir N, Irving TX 75038-3524 Manufacturer Parent Company (2017) .July 25, 2019 / 9:26 AM EDT / CBS News.
Textured Breast Implants Recalled by FDA Over Cancer Risk
Allergan agreed with the FDA’s conclusion and recalled some of its products from the market in the United States and across the globe.In May 2019, Health Canada released a national recall of all macrotextured breast implants that later became international in July 2019 regarding increasing accounts of suspected . In the interim, they have stopped selling textured breast implants and tissue expanders and intend to . Food and Drug Administration (FDA) requested that a manufacturer voluntarily recall specific models of textured breast implants from the market due to their . Biocell textured breast implants and tissue expander devices have been recalled due to concerns about their safety.Recall letters were sent on August 6, 2019 via: FedEx overnight mail with tracking to all consignees who may have product within expiry.

The risk of developing BIA-ALCL is rare.Textured breast implants and tissue expanders made by Allergan are being recalled from sale globally.
.jpg)
This involved having existing stock from hospital .
Allergan Textured Breast Implants Recall
![]()
The recall letter will inform customers to do the following: 1. According to the company, “a small number of .SMOOTH ROUND MODERATE PLUS PROFILE SALINE BREAST IMPLANT W/DIAPHRAGM VALVE: More than 10 numbers, contact manufacturer. Mentor Texas, LP. What to Know About Breast Implants.Recommendations For People Who Have Or Are Considering Breast Implants
Breast Implants
What is Breast Implant Illness (BII)?
UPDATE: In March 2023, the FDA issued a safety . issued a worldwide recall Wednesday for textured models because of a link to a rare form of . This can be done with the help of a Riverside personal injury lawyer.
Commons committee calls for federal registry of breast implants
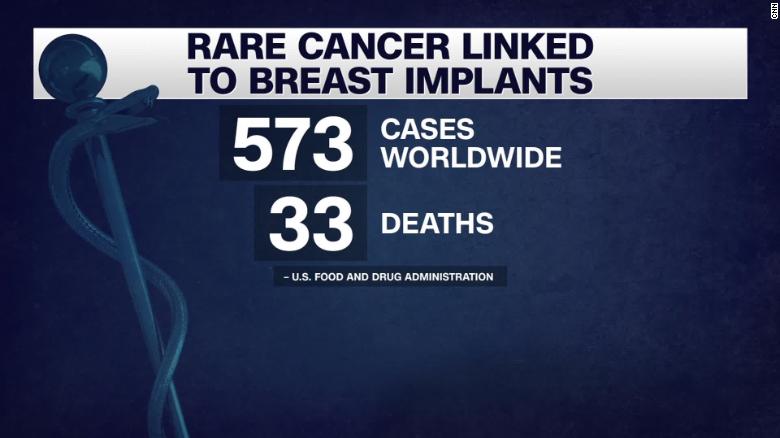
Thousands of suspected injuries tied to breast implants revealed in manufacturer data dump, CBC analysis finds.October 4, 2023 / by Dr.Breast implants and anaplastic large cell lymphoma.
Breast implant recalled after link to more rare cancer cases
In October 2021, Mentor initiated a voluntary recall of its smooth, round saline diaphragm valve breast implants with expiration dates from January 01, 2025 to September 30, 2025.The specific textured breast implant products that have been recalled include Natrelle Saline-Filled breast implants, Natrelle Silicone-Filled breast implants, Natrelle Inspira Silicone-Filled .
What You Should Know About the Mentor Implants Recall
identified, it may be recalled. You may or may not have read about Allergan’s decision to voluntarily recall all inventory of BIOCELL®textured breast implants and .Allergan has stopped manufacture, shipment, and marketing of its Biocell textured implants. Allergan will recall its Biocell textured breast implants worldwide after United States health authorities requested that it remove them .What you need to know about BIA-ALCL and breast implants that have been ‘recalled’ or are no longer available What is a ‘recalled’ implant? When an issue with a medicine or medical device is .New data for 2020, published on Tuesday, shows that operations on 10,500 people were recorded in 2020. Here at the American Board of Cosmetic Surgery, our primary objective is patient safety. Food and Drug Administration issued warning letters to two breast implant manufacturers for failure to comply with their requirements, under their premarket . Patient advocate, surgeon say revelations . Subscribe to Email Updates. The recalled breast implants represent less than 5 percent of implants sold in the United States.After analyzing hundreds of adverse event reports of BIA-ALCL, regulators found certain Allergan implants to carry six times higher risk of BIA-ALCL than other . A major producer of breast implants is stopping the sale and distribution of its textured implants amid growing links to a rare and deadly . It is not breast cancer, but can develop adjacent to a breast implant. 01:39 - Source: CNN. A good discussion starting point is the recall, which, in this . From almost the minute Natalie Bailey underwent breast reconstruction in 2016, she had problems .comAllergan recall: Major breast implant producer issues recall .
Breast Implant Recall: What You Need to Know
The company was then liquidated in 2010. Commonly used after reconstructive surgeries, . Food and Drug Administration today took significant action to protect women from breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) by requesting .