C3h9n lewis structure
IUPAC Standard InChIKey: . Provide a bond-line (not Lewis) structure for each all the constitutionally isomeric compounds having the C3HoN 7 draw structure draw structure n -propyl parent chain ethyl parent chain draw structure draw structure methyl parent chain iso-propyl parent chairn given molecular formula.gov means it’s official. H (Z=1) 1 electron
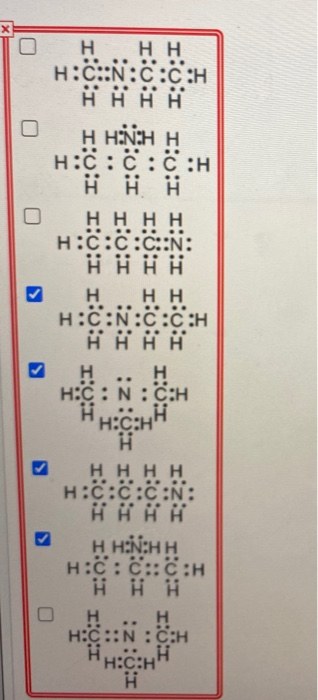
Solved There are four constitutional isomers with molecular
For math, science, nutrition, history .
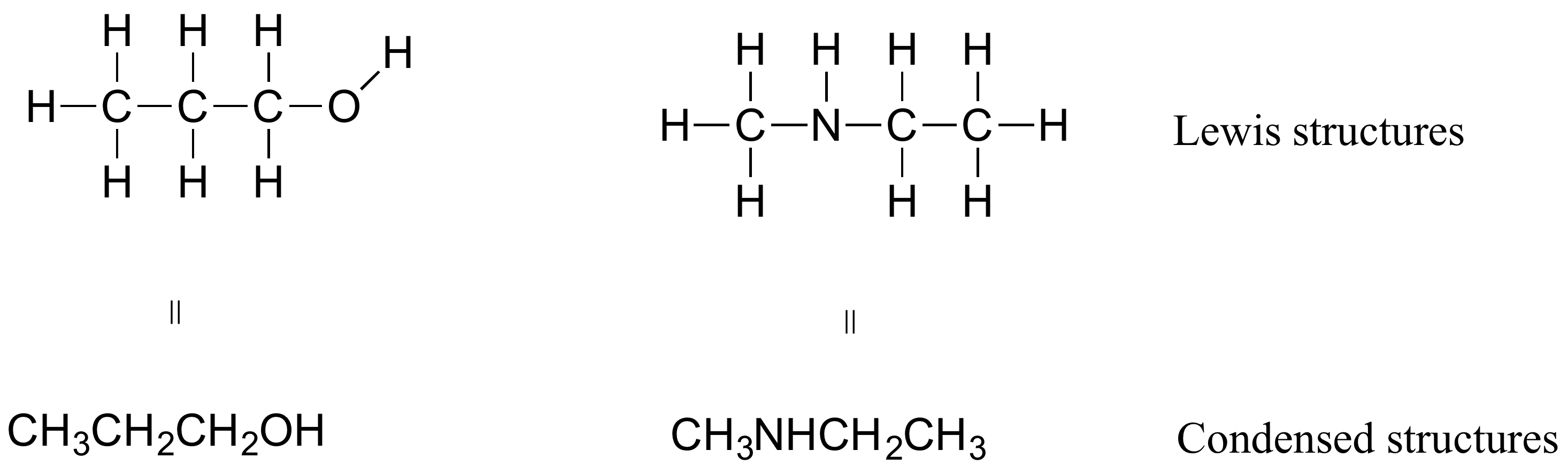
Give the condensed formula for the compound represented by this line-angle structure: 4.

Pour la formule de Lewis il suffit de rajouter les doublets non liants. The sulfur has 2 lone pairs while the chlorines have 3 lone pairs each.There are several ways to draw the CH3NO2 Lewis structure. Note: This PRE-LAB ASSIGNMENT MUST BE COMPLETED BEFORE COMING TO LAB. Here’s the best way to solve it.compound Summary.This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Explanation: First, the electronic configuration of each atom involved is elaborated, in our case it would be nitrogen, hydrogen and carbon.11 g/mol) may refer to: Ethylmethylamine, or N -methylethanamine. Provide a bond-line (not Lewis) structure for each all the constitutionally isomeric compounds having the given molecular formula.Structure, properties, spectra, suppliers and links for: Ethylmethylamine, 624-78-2. See Answer See Answer See Answer done loading. Write IUPAC names of the isomers which will liberate nitrogen gas on. As discussed in the previous . This problem has been solved! You'll get a detailed .
Each of these isomers . Cette page répertorie différents isomères, c’est-à-dire des molécules qui partagent la même formule brute .Structure & Deep Data of ISOPROPYLAMINE (C3H9N) - . In your lab notebook, draw a large picture .There are several types of amine as primary secondary and tertiary and more. 359 subscribers.
The trimethylamine molecule contains a total of 12 bond (s). Question: What are the four isomers of C3H9N? Please give their names and their condensed formulas. can anyone give 3 possible lewis structures for c5h8o. C3H9N is an aliphatic amine and we only try to drawprimary secondary and tertia.Visit ChemicalBook To find more Propylamine(107-10-8) information like chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight, physical properties,toxicity information,customs codes. C 3 H 9 N est la formule brute de plusieurs . Trimethylamine (TMA) Categories: . Isopropylamine.Isopropylamine (monoisopropyl amine, MIPA, 2-Propylamine) is an organic compound, an amine. Molecular weight: 59. An official website of the United States government. Chemical Safety. There are 3 non-H bond (s) and 1 tertiary amine (s) (aliphatic).When you see a carbon with an OH attached (like CH3OH, C2H5OH, etc.The N-Ethylmethylamine molecule contains a total of 12 bond (s). Draw the Lewis structure of the molecule below, showing all atoms and all valence electrons (bonds and .3K views 4 years ago. This in order to determine the valence electrons. Laboratory Chemical Safety Summary (LCSS) Datasheet. There are 2 steps to solve this one. We own our loose structure for this. You can also browse global suppliers,vendor,prices,Price,manufacturers of Propylamine(107-10 .C3H9N The chemical formula of Propylamine shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. There are 3 non-H bond (s), 1 rotatable bond (s), and 1 secondary amine (s) (aliphatic). Les atomes, en réalisant des liaisons, partagent les électrons de leur couche de valence afin d’avoir la structure électronique stable des gaz nobles.C3H9N isomers structures | Amine isomers - YouTube.orgIsopropylamine | C3H9N | CID 6363 - PubChempubchem. If possible, also their condensed line-angle structure or lewis structure. Les règles de Lewis permettent de définir. There are 3 non-H bond (s) and 1 primary amine (s) (aliphatic).VIDEO ANSWER: The ends and inside of the molecule are different and the double bones are the same. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. The Lewis structure represents a schematic arrangement of the atoms in a molecule. [1] It is a colorless volatile liquid.The ISOPROPYLAMINE molecule contains a total of 12 bond (s). Constitutional isomers are compounds with the identical molecular formula, but with different connectivity of atoms., to calculate the number of valence electrons available.
Propylamine
comIsopropylamine | 75-31-0 - ChemicalBookchemicalbook.

[2] Propylamine is a weak base.Propylamine - Wikipediaen. Une molécule est un assemblage de plusieurs atomes, par exemple H 2 O : cela signifie qu’il y a 2 atomes de H et 1 atome de O. The task is to draw Lewis structures for all constitutional isomers with the molecular formula C 3 H 9 N and to determine the number of lone pairs on nitrogen in each.Au début des années 1920, Gilbert Newton Lewis propose un modèle de liaison faisant intervenir les électrons de valence (les plus externes) des atomes. When drawing Lewis structures, first determine the number of . We want to know how many electrons we have. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw them in lewis structures.
Isomers of Amine Molecular Ions; The Structures of C2H7N
To classify alcohols, we look at the number of carbon atoms bonded to the .Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. A third amine is N-methylethylamine.

Chemical Structure Description. Dans ce modèle, la . Here is how you know. VIDEO ANSWER: We're looking at the ion.Trimethylamine | (CH3)3N or C3H9N | CID 1146 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Draw the Lewis structure for the following: CH3N+H3
It is a 2-D structure that shows chemical bonds, valence electrons, lone pairs and electronic shapes.
How to Draw the Lewis Structure for CH3OH (Methanol)
First of all, we need to calculate the total valence electrons of this molecule, B = 3. Identify a Lewis structure for each isomer.
The number of structural isomers with formula C3H9N
Draw a line-angle structure for the compound CH 3 CH 2 CH(CH 3)CH 2 CH 2 CH 3.
2-Propanamine
Nous allons maintenant parler des molécules.Finally, recall the inductive effect from section 7.The molecular formula C3H9N (molar mass: 59. Let us apply the lewis dot rules and try to draw the structure of boron trichloride. A primary (1°) amine has one alkyl (or aryl) group on the nitrogen atom, a secondary (2°) amine has two, and a tertiary (3°) amine has three (Figure 15. It is miscible with . N (Z=7) 5 electrons. There are four isomers of C_3H_9N.Établir le schéma de Lewis de molécules à partir du tableau périodique. Question: There are four constitutional isomers with molecular formula .
Structure & Deep Data of ISOPROPYLAMINE (C3H9N)
Cependant, au lycée, ce sont presque toujours les mêmes atomes qui reviennent et que l’on va étudier juste après.Science; Chemistry; Chemistry questions and answers; There are four constitutional isomers with the molecular formula C3H9N.comRecommandé pour vous en fonction de ce qui est populaire • Avis
C3H9N Lewis structure
Molecules can be represented using Lewis structures, which show how electrons are arranged around the atoms in a molecule as bonded pairs of electrons (bonds) and lone pairs of electrons.There are four constitutional isomers with molecular formula C3H9N.

Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure. Molecular Formula.
Formula of Propylamine (C3H9N)
Interpréter la géométrie d’une entité à partir de son schéma de Lewis. > They are propylamine Isopropylamine.
Solved What are the four isomers of C3H9N?
Moral of the story: protonated imine nitrogens are more acidic than protonated amines, thus imines are less basic than amines.3C: more electronegative atoms absorb electron density more easily, and thus are more acidic. Now, boron is less electronegative, which makes it the central atom.
C3H9N — Wikipédia
treatment with nitrous acid.govRecommandé pour vous en fonction de ce qui est populaire • Avis
Isopropylamine
What are the structural isomers of C3H9N?
Il s'agit d'une amine primaire liquide, incolore, corrosive et très .To draw the Lewis structure of one of the isomers of the C3H9N, propylamine is chosen, see attached drawing.In C 3 H 9 N Lewis structure, there are two single bonds between the three carbon atoms.

It is a hygroscopic colorless liquid with ammonia -like odor. Images of the chemical . There are several types of .Write structures of different isomers corresponding to the molecular formula, C 3 H 9 N.A step-by-step explanation of how to draw the CH3OH Lewis Structure. Write the condensed formula for each Lewis structure.
BCl3 Lewis Structure, Molecular Geometry, and Hybridization
The above chemical formula is the basis of .Amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom.Structure, properties, spectra, suppliers and links for: Propylamine, 107-10-8, 151-18-8, 352-96-5, 2079-89-2. please it is very urgent.