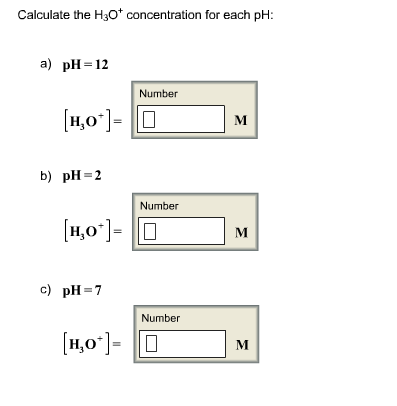
Calculate h3o and ph

Calculate the [H3O+] and pH of each of the following H2SO4 solutions.10 M sodium acetate.Use pH to calculate [H+] and pOH to calculate [OH-] = .
H3o+ Concentration Calculator Online
The mathematical form of the pH is the following.In chemistry, pH (denoting “potential of hydrogen” or “power of hydrogen”) is a quantitative measure used to specify the acidity or basicity of an aqueous solution. See Answer See Answer See Answer done loading.This video is about the 3 equations you need to memorize to solve problems dealing with pH. And so, at this temperature, acidic solutions .The calculation of pH becomes straightforward.This quiz helps you practice calculating pH and pOH from hydrogen ion (H+) and hydroxide ion (OH–) concentrations and vice versa.31, acidic solutions exhibit pH less than 6.20Critiques : 10 ×10−7 M 1.
(Milk of magnesia is largely Mg (OH) 2 .8 can be rewritten as. pure water, pH = 7. An antilog is how you would undo a logarithm by making both sides. In order to calculate pH, we must take the common (base 10) logarithm of .If we wish to find the hydronium ion concentration ([H 3 O +]) and the pH of a solution, we need to know both the strength of the acid (or base) and the concentration of the acid (or base). This webpage is part of a . These acids are often used in industry and everyday life. How are pH and H3O+ concentration related? If the hydronium .What is the correct way to calculate the concentration $\ce{H3O+}$ in a solution with $\ce{pH}=6. The most important of these is undoubtedly the H 2 CO 3 /HCO 3 – pair, but side chains of the amino acid histidine in the hemoglobin molecule also .
How to Calculate pH
How do you calculate H+ from pH? In the following equation, pH = −log [H+], where [H+] denotes the molar hydrogen ion concentration.0001 moles per liter. pH Practice Problems. To illustrate how this calculator works, let’s consider a solution with a pH of 3. pH<7, therefore there are only $\ce{H3O+}$ particles in the solution.Calculate [H3O+] and the pH of each H2SO4 solution (Ka2=0.2024 Thesis Committee Meeting. Calculate the [H3O+] for 0.And we have the pOH equal to 4. Grâce à ce calculateur de pH, on peut déterminer le pH d'une solution de plusieurs manières.00 L of solution? C) What must be added to a solution of sodium acetate in . It can also be stated that the pH is a negative log of the molar concentration of hydronium ion [H 3 O + ]. In this set of practice problems, we will work on examples correlating the acidity and basicity of a solution with pH, calculating the pH of strong and weak acids and bases, the pH and pOH relationship, and .
pH Calculations
Stack Exchange Network.We can then find the pH. Skip to main content. So we all know that p h equals negative log age plus so H plus orange three old plus equals ten to the negative pH equals turn to the negative eight point five five, which equals good point eight to times ten to the leggo of knots and also h three o plus times.Transition Metals and Coordination Compounds 3h 14m. Q: Calculate the concentration of H+ in an aqueous solution with a pH of а.31 and pOH less than 6.
Determining and Calculating pH
Asked for: \(pK_w\), \(pH\), and \(pOH\) Strategy: Calculate \(pK_w\) by taking the negative logarithm of \(K_w\). A. Here it helps to rewrite the .Example of H3O+ Concentration for Each pH Calculator. With a pH greater than 7, milk of magnesia is basic.8 × 10 −5-M solution of HCl). The pH and pOH of a neutral solution at .Solve for the concentration of \(\ce{H3O^{+}}\) using the equation for pH: \[ [H_3O^+] = 10^{-pH} \] Use the concentration of \(\ce{H3O^{+}}\) to solve for the concentrations of the . If the temperature changes then the Kw also changes which means what cons.
![Solved Calculate pH for each H3O concentration: a) [H3O ] | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media/26a/26aa5457-1e32-4c97-ae53-d6c153f5ea58/phpSXY2bm.png)
[ H X +] = − log. Notice that we are required to take the common (base 10) logarithm of the hydrogen ion concentration in order to calculate pH. That only applies at 25°C when the autoionization constant of water, Kw, is equal to 1. The equation also shows that each increasing unit on the scale decreases by the factor of ten on the concentration of H +.The pH of an aqueous solution can be calculated based on the hydronium ion concentration using the equation pH=−log[H3O+]. The thesis committee meets once a year and submits a report that will determine whether or not the PhD student is authorized to . The volume of the final solution is 101 mL.Acids and Bases. This calculation reveals that the hydronium ion concentration is 0.
It's easy to do this calculation on any scientific .0 × 10 − 7) = 7. Il peut convertir le pH en H+, ainsi que calculer le .
![Calculating pH, pOH, [OH] and [H3O] - Real Chemistry - YouTube](https://i.ytimg.com/vi/Zv8hBKKnVnI/maxresdefault.jpg)
Calculate [H3O+] for a 0. Label each solution as acidic, basic, or neutral based only on the stated pH p H.3, the titration curve for NH3, a weak base, is the reverse of the titration curve for acetic acid. The hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 − 7M at 25 °C.Calculate the pH from the equation: pH = -log[H +] = -log[0. This online quiz is intended to give you extra practice in calculating pH and pOH from hydrogen ion (H+) and hydroxide ion (OH-) concentrations and vice versa.Excuse me, how do you convert the 10^-4.00 because the titration produces an acid. Question: Calculate the [H,O*] and pH of each polyprotic acid solution.Answer: pH = -log 10 [H +] pH = -log 10 (1.4 x 10 -5) pH = 4. Use the pH equation \(pH = . The relationship between acid strength and the pH of a solution. pH is defined as the negative decimal logarithm of the hydrogen ion activity, aH+, in a solution: pH = −log10(aH+).The pH is defined as a log of reciprocal of the molar concentration of hydronium ions [H 3 O + ].Determine the pH of acidic and basic solutions. We will find that we need to treat strong acids (and bases) differently than weak acids (and bases) based on the extent to which they react with water.How do you find the pH of a solution that contains a weak acid, a weak base, or a salt? This webpage explains the general method of calculating the pH of weak acids, bases, and salts, using equilibrium constants and ICE tables.055 M HBr, HBr is a strong acid [H 3 O +] = 5. Calculate the [H3O+] and pH of each polyprotic acid solution. The hydrogen ion activity, in turn, depends on the .6, the results are usually fatal.350 M H2C204 ; This problem has been solved! pH = log 1 [H3O+] = − log[H3O+] p H = log. Use the pH equation which is: \(pH = -\log[H_{3}O^+]\). The pH and pOH of a neutral solution at this temperature are therefore: pH = − log[H3O +] = − log(1. There are two calculators – one for either strong acid or strong base, and another for either weak acid or weak base. Select your preferences below and .
Worked examples: Calculating [H₃O⁺] and pH
Is the solution acidic, basic, or neutral? Why? B) HBr is a strong acid.If the pH of human blood, for instance, gets outside the range 7. A neutral pH is only true at 25°C because Kw 1.The calculation for determining the concentration of hydronium ions ( [H3O+]) in a solution is based on the negative base-10 logarithm of the pH value: [H3O+] .The pKb of ammonia is 4.001 M, demonstrating the tool’s practicality in converting pH values to H3O+ concentrations . As shown in part (b) in Figure 17.so at the end (last problem), the PH value for neutral (equal concentration of H+ and OH-) water @ 5. You will also learn how to use approximations and assumptions to simplify the calculations.
And solving for the pH, we get that the pH . That gives us pH plus 4. Calculate pH for 0.10 M NaOH is added to 100 mL of a solution of an unbuffered solution with a pH of 4.6\) As we determine the pH of the solution, we realize that the OH-gained using the second ionization constant is so insignificant that it does not impact the final pH value. Learn how to calculate the hydronium ion for a given pH.4\) \(pH = 14 - 4. milk of magnesia, pH = 10.Q: Calculate the [ H3O+ ] and [ OH− ] of a sodium hydroxide solution with a pH = 12. The first one calculates the pH of a strong acid . The pH of blood is controlled by the buffering action of several conjugate acid-base pairs.Calculate the [H,O*] and pH of each polyprotic acid solution.Calculate [H3O+] for a 0.comHow to calculate the concentration H3O+ in a solution with . Express your answer using two decimal places b. The concentrations of acids and bases are often expressed in terms of pH, and as an . In particular, the pH at the equivalence point in the titration of a weak base is less than 7.Calculating the pH of a strong acid or base solution. Calculate the pH of an acetate buffer that is a mixture with 0. Then use the formula which shows the relationship between pH and pOH: pH + pOH = 14. Using our formula: [H3O+] = 10^-3. 10 − pKw = 10 − 14. Below you can find two calculators that you can use to check answers to chemistry problems. There are 4 steps to solve this one.Acids and bases that are completely ionized when dissolved in water are called strong acids and strong bases There are only a few strong acids and bases, and everyone should know their names and properties.8 x 10^-5? For reference, it's around the timestamp . pOH = − log[OH −] = − log(1.So for first, one p h equals eight point five five. Express your answer using two significant figures.0 × 10 − 7 M at 25 °C.Chemistry questions and answers.At this temperature, then, neutral solutions exhibit pH = pOH = 6.99$? Attempt 1.If you mean how does he solve the equation around that time, he's using antilog.500 mol of HBr in sufficient water to make 1.125K views 7 years ago General Chemistry 2. [ H X 3 O X +] You can . For a neutral aqueous solution, \([H_3O^+] = .75, so we can plug that into our equation. For basic solutions, you have the concentration of the base, thus, the concentration of the hydroxide ions OH-.For comparison, calculate the pH after 1. Find the pH if the H + concentration is 0.So if the neutrality point depends on temperature, does that mean the entire pH scale depends on tem.31, whereas basic solutions exhibit pH greater than 6.Recommandé pour vous en fonction de ce qui est populaire • Avis
Calculateur de pH
Calculate the pOH by plugging the \(\left[\mathrm{OH}^-\right]\) into the equation. \(pOH = -log(4 \times 10^{-5}) = 4.
![pH Calculations - Calculate [H3O ] and [OH-], and Find the pH of a ...](https://i.ytimg.com/vi/v67bWn-ogkE/maxresdefault.jpg)
Calculate the pH by rearranging and plugging in the pOH: pH = 14.Question: 1) A) The [OH-] in a solution is 8.
Wolfram
As was demonstrated previously, the hydronium ion molarity in pure water (or any neutral solution) is 1.So pH + pOH does not always sum to 14?Correct.pH pH by definition is the negative logarithm of hydronium ion concentration.