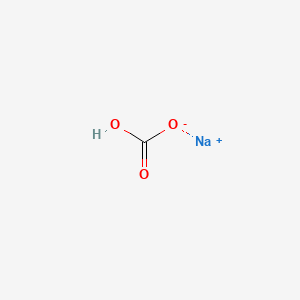
Chemical structure of sodium bicarbonate

The chemical is created on a large scale by reacting cold and concentrated brine (sodium chloride) solutions with CO2 and ammonia.Three balloons are attached to three bottles as shown in the picture. It is added to food and used to de-ice roads in winter. Acidity (pKa): 10. Appearance: White crystalline solid. It is a general chemical compound by classification.0066 g/mol Density: Solid Form – 2. Data from NIST Standard Reference Database 69: NIST Chemistry .
The Chemistry of Baking Soda. The molecule is formed by the sodium cation Na + and . It was first produced in 1791 by the French chemist Nicolas Leblanc and was introduced to the US in the 1840s by John Dwight and Austin Church.sodium bicarbonate (NaHCO3), white crystalline or powdery solid that is a source of carbon dioxide and so is used as an ingredient in baking powders, in . It releases carbon dioxide .comSodium Bicarbonate Facts – Baking Soda or Sodium . The active ingredients of Gaviscon (Ph Eur stands for European Pharmacopoeia, a compendium of pharmaceutical ingredients used in Europe).Sodium carbonate () is known to be a disodium salt of carbonic acid ). A sodium cation (Na+) and a bicarbonate anion (HCO3) combine to form this salt. Pile of Sodium bicarbonate powder. This is because it’s composed of sodium ions (Na+) and bicarbonate ions (HCO 3– ).Vue d’ensemble
Sodium bicarbonate
It is a chemical compound, sodium hydrogen carbonate, with the formula NaHCO 3.
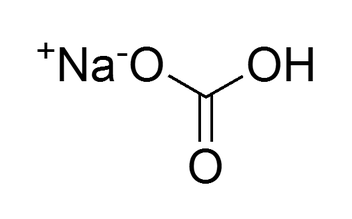
Sodium Bicarbonate (NaHCO3) - Definition, Structure, .comSodium bicarbonate - SpectraBasespectrabase. Anne Helmenstine. Sodium bicarbonate is considered one of the oldest leavening agents used in baked goods.It is a salt composed of a sodium cation (Na+) and a bicarbonate . Molecular Structure of Sodium Bicarbonate. The elements within it are sodium, hydrogen, carbon, oxygen. IUPAC Standard InChIKey: . It’s a powdered variant of a crystalline white solid chemical . Oscillation rheology, elongational viscosity, and extensibility of doughs were tested to evaluate the effect of salt and baking soda on the physical properties of doughs.This is the structure of sodium bicarbonate, also known as sodium hydrogen carbonate or baking soda.173 g·cm -3, melting point of 50 ºC, and decomposes at .6,752 sodium bicarbonate stock photos, 3D objects, vectors, and illustrations are available royalty-free. It exists as a white crystalline solid.Chemical properties.It is a polyatomic anion with the chemical formula H C O − 3. Citrosodina Neut Bicarbonate Of Soda Sodium Hydrogen .comRecommandé pour vous en fonction de ce qui est populaire • Avis
Sodium bicarbonate
Sodium bicarbonate or baking soda isolated on white background.
Sodium bicarbonate Formula
Sodium bicarbonate is highly soluble in water and slightly soluble in alcohol.For NaHCO3 we have an ionic compound and we need to take that into account when we d.comSodium Bicarbonate (NaHCO3) - Structure, Properties, Usesbyjus. Sodium bicarbonate is a white, crystalline substance that is commonly .
Sodium bicarbonate: Molecular Geometry
The Solvay technique used to . The third bottle contains 100 ml of water and 150 ml of a soft drink.The most common compound of sodium is sodium chloride (common salt).In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid.Date : Not specified, most likely prior to 1970
Sodium Bicarbonate (NaHCO3)
Formula: CHNaO 3.From a chemical perspective, sodium bicarbonate is a type of salt, an ionic compound composed of sodium ions (Na+) and bicarbonate ions (HCO 3– ).Sodium bicarbonate, with the chemical formula NaHCO3, is the white powder widely known as baking soda. A similar compound is sodium carbonate (Na2CO3), used as a cleaning agent or an additive during clothes washing. It is mildly alkaline in nature and is often used to neutralize acids. Le bicarbonate de sodium est un sel formé par la . See sodium bicarbonate stock video clips. All of the balloons contain Alka-Seltzer. The molecular formula of sodium bicarbonate is NaHCO3. Carbonate is a polyatomic anion with the formula CO2−3 C O 3 2 − and has a trigonal planar molecular structure which consists of a carbon atom surrounded by three oxygen atoms.
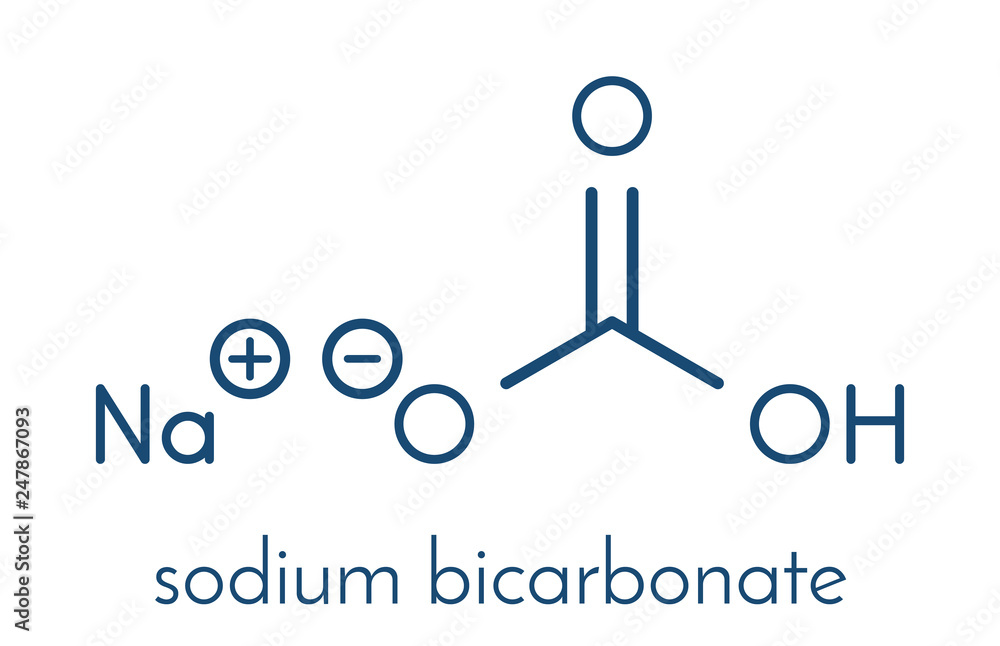
Structure, properties, spectra, suppliers and links for: Sodium bicarbonate, 144-55-8, NaHCO3. The second bottle contains 250 ml of water and 3 ml of HCl.Properties of Sodium Bicarbonate: Chemical Formula: NaHCO 3 Molecular Weight/Molar Mass: 84. 3 and a molecular mass of 61. Sodium carbonate is water-soluble and forms an alkaline solution as it is composed of salt of a strong base and weak acid. It is used in the kitchen for various uses, including as an ingredient that makes dough rise or for removing odors in the refrigerator. This activity outlines and reviews the indications, action, and contraindications for sodium bicarbonate as a valuable agent in the treatment, management, and therapy . These are facts, chemical properties and physical properties of sodium bicarbonate, which is also known as baking soda or sodium hydrogen carbonate. In both chemistry and baking, sodium bicarbonate is considered a base because it creates a reaction when mixed with acids, like buttermilk, yogurt or vinegar. Sodium Bicarbonate. At its core, sodium bicarbonate’s chemical formula, NaHCO₃, encompasses sodium, a reactive alkali metal, hydrogen, a diatomic non-metal gas, carbon, a versatile non-metal, and oxygen, a vital life-supporting element. JEE Exam JEE Study Material Chemistry NaHCO3.Molecular Structure of Sodium Bicarbonate. Molecular Weight: 84. It is used as a water softener.Sodium bicarbonate. Sodium bicarbonate, which is categorized as an antacid, also makes your stomach's environment less acidic (more .Formula and structure: The sodium bicarbonate chemical formula is NaHCO 3 and its molar mass is 84.Sodium bicarbonate is a medication used in the management and treatment of multiple disease pathologies. It’s also named as baking soda as on heating it produces bubbles of carbon dioxide which . Le bicarbonate de soude est le .01 daltons; it consists of one . Sodium bicarbonate, commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO 3.
Sodium Bicarbonate (NaHCO3): Properties, Structure, and Uses
Its scientific formula is NaHCO 3 and it falls into the category of a salt. Nicolas Leblanc, a French chemist, invented sodium carbonate in the year 1791. Furthermore, a series of physical–biochemical analytical techniques were . Sodium bicarbonate is baking soda, bicarb or bicarbonate of soda.Sodium bicarbonate is an inorganic compound with the chemical formula NaHCO3 N a H C O 3. Drop the alka-seltzer into the bottles. This reaction results in the liberation of carbon dioxide gas.Soft wheat flour doughs were prepared with different levels of salt (NaCl) or baking soda (NaHCO3). The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula HCO−.Overview
Sodium bicarbonate
La formule chimique du bicarbonate de sodium, également connu sous le nom de bicarbonate de soude, est NaHCO3.Chemical Composition and Structure.sodium bicarbonate (sodium hydrogencarbonate); and calcium carbonate. Sodium bicarbonate or baking soda on wood background.Auteur : Wayne BreslynNa/c2-1 (3)4;/h (H2,2,3,4);/q;+1/p-1.
Sodium Bicarbonate
orgSodium bicarbonate - NISTwebbook. Upon heating, it undergoes decomposition to form .The structure of the molecule also plays an important role in determining polarity, state of matter, colour, magnetism, taste, and many other properties.
Sodium Bicarbonate: Uses, Side Effects, and More
It is also used as a feedstock for the chemical industry. In its pure form, soda ash exists as a white powder and is known to be odourless.Sodium bicarbonate Sodium bicarbonate chemical structure.C'est la formule chimique du bicarbonate de soude, montrant ses ions dans l'eau.Sodium Bicarbonate Molecule-- Space Fill Model To View the Sodium Bicarbonate in 3D using Jsmol.Sodium Bicarbonate = Sodium Oxide + Carbon Dioxide + Water.Four moles of Sodium Bicarbonate [NaHCO 3] and one mole of Acetic Acid [CH 3 COOH] react to form four moles of Sodium Formate [NaCOOH], two moles of Carbon Dioxide [CO 2] and two moles of Water [H 2 O] Show Chemical Structure Image.Explanation (including important chemical equations) HCO 3- (aq) + H + (aq) H 2 CO 3 (aq) H 2 O (l) + CO 2 (g) HCl is a stronger acid than water. It is composed of an ionic bond between two sodium ions and a . Hybridization : Hybridization in chemistry is the process of mixing, merging, or combining two or more different orbitals of electrons in the same atom.Chemical structure: This structure is also available as a 2d Mol file; Species with the same structure: Sodium bicarbonate; Sodium hydrogen carbonate; Information on this page: Notes; Other data available: IR Spectrum; Options: Switch to calorie-based units; Notes.Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3 and the IUPAC designation sodium hydrogencarbonate.Structure: CAS Number: 144-55-8.Sodium bicarbonate, commonly known as sodium hydrogen carbonate, has a monoclinic crystalline structure. Chemical Formula: NaHCO3. Sodium bicarbonate . The carbonate ion is a moderately strong base, so by definition of a Lewis base, it attracts protons in aqueous solutions. Mis à jour le 08 mars 2019.Baking soda, chemically known as sodium bicarbonate (NaHCO 3) has a simple chemical structure, which consists of sodium ions (Na +) and bicarbonate ions .¹, ² Today, baking soda is a widely used chemical leavening agent due to its accessibility . This chemical reaction produces carbon dioxide (CO 2) in the form of bubbles, like a liquid foam.govSodium bicarbonate | Chemistry Onlinechemistry-online. Sodium bicarbonate is an antacid. more acidity yields more CO 2 gas).
NaHCO3
Sodium
Baking soda makes baked foods rise, but the chemical has a lot of .The main physicochemical properties of NaHCO 3 are as follows: molar mass of 84 g·mol -1, density of 2. Sodium Bicarbonate - NaHCO 3.Regarder la vidéo2:46A step-by-step explanation of how to draw the NaHCO3 Lewis Dot Structure.Sodium bicarbonate is a natural chemical substance, which is also known as baking soda.
Structure, Uses, Health Effects of Bicarbonates with FAQs
The term bicarbonate was . Larger amounts of acid will increase the acidity of the solution and push the reaction towards the production of products (i. It is a white crystalline powder.
NaHCO3 + CH3COOH = NaCOOH + CO2 + H2O
In 1846, two New York bakers, Austin Church and John Dwight, established the first baking soda plant.Sodium Bicarbonate | NaHCO3 or CHNaO3 | CID 516892 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, . The bicarbonate ion is the .Na2CO3 (aq) + H2O + CO2 (g) → 2 NaHCO3 (aq) The chemical compound sodium carbonate exhibits a propensity to engage in chemical reactions with acids, including those of a mild nature such as those found in vegetable sources, for instance, lime juice. The crystalline structure of sodium bicarbonate, also known as sodium hydrogen carbonate, is . Biological role. IUPAC Standard InChI: InChI=1S/CH2O3. This white solid crystalline substance has diverse applications, . It is odorless, has a salty, alkaline taste, and is soluble in water.comRecommandé pour vous en fonction de ce qui est populaire • Avis