Homogeneous mixture definition
Each of the layers is called a phase.
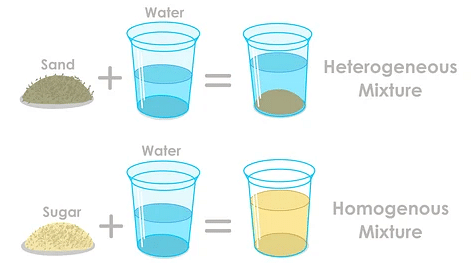
A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture.By definition, a pure substance or a homogeneous mixture consists of a single phase. Examples are air, saline .A homogeneous mixture is one that contains the same proportions of its components in a given amount of sample. Example \(\PageIndex{1}\) Identify each substance as a compound, an element, a . A phase is any part of a sample that has a uniform composition . Example: Air is a homogeneous mixture of gases. Heterogeneous mixtures may contain a homogenous mixture as one .

These are mixtures of different elements. In other words, a mixture is a material that contains particles of two or more pure substances in any proportion. It is uniform in . A heterogeneous mixture is a mixture with a non-uniform composition.A heterogeneous mixture is a non-uniform composition of two or more ingredients or phases.
Heterogeneous Mixture
Mixtures can be either homogeneous or heterogeneous: a mixture of uniform composition and in which all components are in the same phase, such as salt in water, is called . In compounds, atoms of various elements .Suspensions definition Heterogeneous mixtures vs. Such materials can be compounds or chemical elements. Solutions are composed of a solvent (major component) and a solute (minor component).2: Solutions: Homogeneous Mixtures is shared under a CC BY-NC-SA 4.1: Homogenous Mixtures is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Examples of mixtures include air, salt water, .

En savoir plus. The particles are too small to see and also too small to settle or be filtered out of the mixture.
Manquant :
definition They have the same appearance and chemical composition throughout. All of them share the same physical attributes and chemical composition. It is formed when elements or compound or both mix together . The prefix ‘homo’ means same and indicates that the two substances combined .Mélanges hétérogènes et homogènes : quelle est la différence
Learn the difference between homogeneous and heterogeneous mixtures. Examples of Homogeneous Mixtures include Water, Air, Steel, Detergent, Saltwater mixture, etc. Mixture of Oil and Water is a heterogeneous Mixture. two or more substances that have mixed.
Manquant :
definitionHomogeneous and Heterogeneous Mixtures
0 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry . The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample.Un mélange homogène est un mélange dans lequel les composants qui composent le mélange sont uniformément répartis dans tout le mélange.Provides the definition of mixture and homogeneous mixture. A homogeneous mixture is a mixture in which all components are uniformly distributed, and all samples appear the same. Example: rainwater, vinegar, etc. “Like dissolves like” is a useful rule for deciding if a solute will be soluble in a solvent. The composition varies from one region to another with at least two phases that remain separate from each other, with clearly identifiable properties. Please update your bookmarks accordingly.20 Examples of Homogeneous mixtures – LORECENTRALlorecentral. When oil and water are combined, they do not mix evenly, but instead . The composition of the mixture is the same .comRecommandé pour vous en fonction de ce qui est populaire • Avis Another word for a homogeneous mixture is a solution.
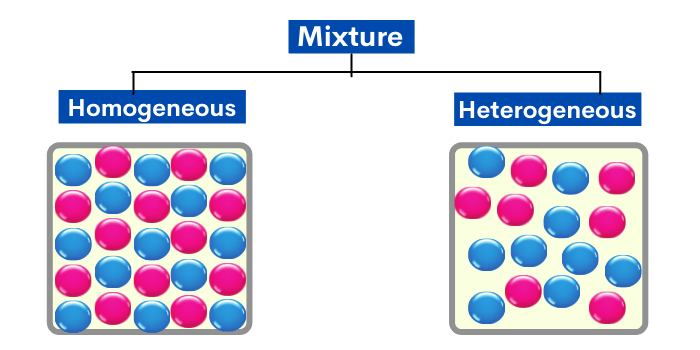
Homogeneous mixtures can be solid, liquid, or gas. It exhibits uniformity in appearance and properties throughout the mixture.By combining two or more substances, a mixture is produced.A homogeneous mixture is a combination of two or more substances that is so intimately mixed, that the mixture behaves as a single substance.The homogenous mixture/solution has only one phase that is solid, liquid and gas. Click here to view We have moved all content for this concept to for better organization.org10 Heterogeneous and Homogeneous Mixtures - ThoughtCothoughtco.Uniform composition. Definition of Mixture: In chemistry, a mixture is a substance that contains two or more substances, either elements or compounds or both in any ratio. However, a heterogeneous mixture has . A homogeneous mixture is characterized by a uniform composition, where the components are evenly distributed at the molecular level. It is different from a pure .
Homogeneous & Heterogeneous Mixture

Marisa Alviar-Agnew ( Sacramento City College) Henry Agnew (UC Davis) 13.
Homogenous and Heterogenous Mixtures
When oil and water are combined, they do not mix evenly, but instead form two separate layers. 1: Solutions, Suspensions, and Colloids.There are two types of mixtures: mixtures in which the substances are evenly mixed together (called a homogenous mixture, or solution) and a mixture in which the . Pure substances are those in which all the molecules are of the same .We are able to recognize individual particles by looking at a mixture. If you examine a sample of a heterogeneous mixture, you can see the separate components. Mixture of Salt (Sodium Chloride) and Iron filings is a heterogeneous mixture. Mixtures can be solids, liquids, gases, or a combination of states of matter.homogeneous mixture définition, signification, ce qu'est homogeneous mixture: 1.Choose 1 answer: These are mixtures of different elements. When the salt is thoroughly mixed into the water in this glass, it will form a solution.A mixture in which the compounds are uniform in the whole solution, its formation in the whole solution is the same and appears like a single substance is called a homogeneous mixture.HOMOGENEOUS MIXTURE. Learn more about the meaning, .Homogeneous Mixture Definition.A homogeneous mixture is a physical blend of two or more components, each of which has the same composition and properties. Saltwater: The combination of salt and water forms a homogeneous mixture where the salt molecules . Gas homogenous mixture: air is a mixture . Liquid homogeneous mixture: a saline solution that is the mixture of water and salt.Henry Agnew (UC Davis) 8. Any given spoonful of soup will contain varying amounts of the different vegetables and other components of the soup. A heterogeneous mixture consists of two or more phases.On February 21, 2024.Learn the definition of homogeneous and heterogeneous mixtures, and see examples of each type.
What are Homogeneous Mixtures?
We should not confuse a homogenous mixture with a compound. Homogeneous Mixture is a substance composed of two or more components that are uniformly distributed at the molecular or microscopic . We are able to see oil and water clearly separately in the mixture.
Homogeneous Mixtures
In addition, uniform mixture is another term for homogeneous mixture and non-uniform mixture is another term for heterogeneous mixture. In simpler terms, it appears the same throughout, exhibiting a seamless blend of substances.0 license and was authored, remixed, and/or curated by LibreTexts. When oil and water are . Watch a video and read the comments from other learners on Khan Academy. The salt water described above is homogeneous because the dissolved .A homogeneous mixture is a combination of two or more materials that appears uniform, regardless of where you sample it. Examples of mixtures include .What is a homogeneous mixture? Define homogeneous mixture in chemistry.A mixture is a material composed of two or more simpler substances in chemistry. They can be separated by evaporation.A homogeneous mixture is a type of mixture where two or more substances have mixed completely and are the same throughout. Sand, oil and water, and chicken noodle soup are examples of heterogeneous mixtures.A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. The particles of salt and Iron filings can be . Sugar and water, for example, constitute a homogeneous combination because the taste of the .
Wikipedia
A heterogeneous mixture is a mixture in which the composition is not uniform throughout the mixture.
What is a Mixture
Types of mixtures (video)
What is a Heterogeneous Mixture? This is a type of mixture in which all the components are completely mixed and all . These terms are derived from the idea that a homogeneous mixture has a uniform appearance, or only one visible phase, because the particles are evenly distributed. Review examples. There are two types of mixtures: mixtures in which the substances are evenly mixed together (called a homogenous mixture, or solution) and a mixture in which the substances are not evenly mixed (. Examples for the same are; Solid homogeneous mixture: brass is an alloy that is made from metal copper (Cu) and zinc (Zn). homogeneous mixtures.Last Updated : 12 Dec, 2023. To better organize out content, .Definition and Examples. Definition of the homogeneous mixture– Homogeneous mixture is the one, in which the components are uniformly distributed throughout its volume and cannot be seen separately.2: Solutions - Homogeneous Mixtures is shared under a CC BY-NC-SA 3. Homogeneous mixtures might be solid, liquid, or gas.Here, a homogeneous mixture is one in which all components are in a single phase, while a heterogeneous mixture contains components in different . A homogeneous mixture has uniform composition and state of matter, . They usually are homogeneous .A homogeneous mixture has a uniform composition, while a heterogeneous mixture has a non-uniform composition. Pure substances are those in which all the molecules are of the same nature. Often it is easy to confuse a homogeneous mixture with a pure substance because they are both uniform. Figure \(\PageIndex{1}\): Oil and water do not mix, instead forming two distinct . Homogeneous mixtures are sources of water, saline solution, some alloys, and bitumen. When sugar is put in water, for example, it forms a mixture, then it dissolves to create a solution. We have a new and improved read on this topic.