Hydronium ion example

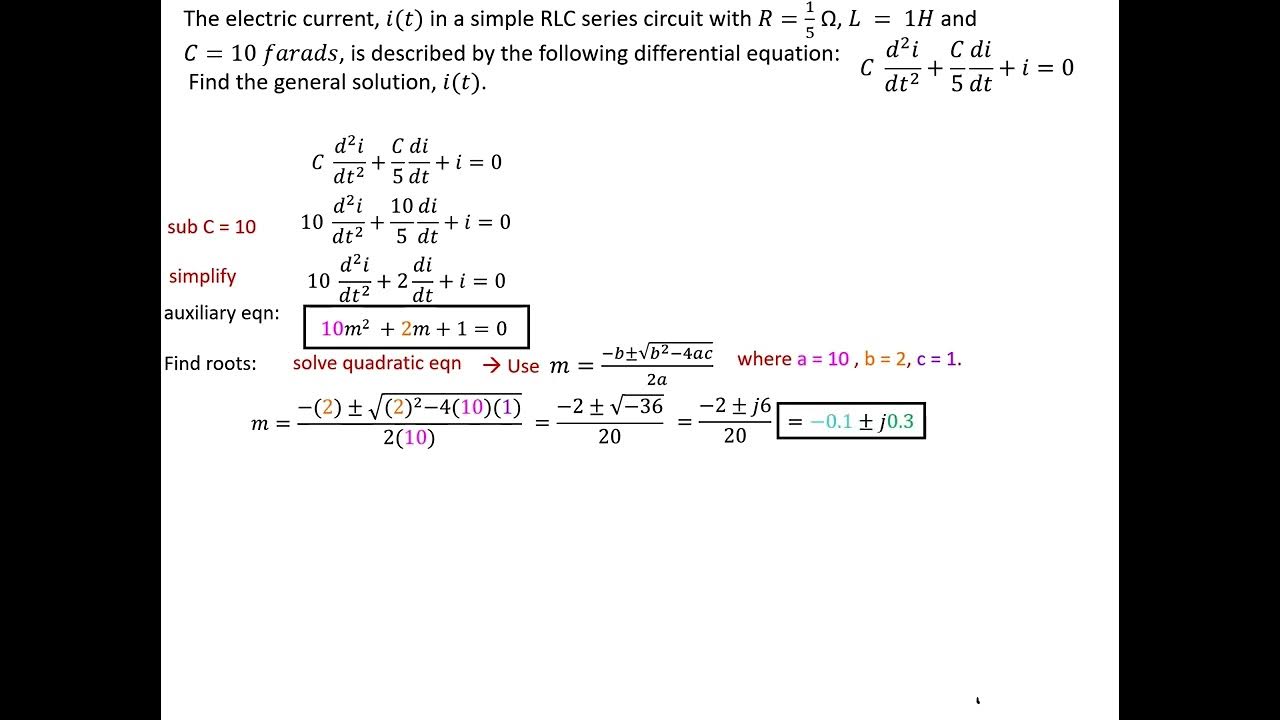
Learn about the hydronium cation, which has the chemical formula H3O+. In this video, we'll solve for [H₃O⁺] and pH in two different worked examples.1, we have not shown the additional water from the hydronium ion and we have grouped the sodium and chloride ions as NaCl (aq), with the understanding that it will be fully ionized in aqueous solution.
Water autoionization and Kw (article)
Hydronium is what you get when you put water and hydrogen ions together, forming H 3 O +.An Arrhenius base is any species that increases the concentration of OH −. The examples include cations like ammonium ion ( NH+4 NH 4 + ), and hydronium ion ( H3O+ H 3 O + ); and anions like hydroxide ion ( OH− OH − ), and cyanide ion ( CN− . Also, general chemistry professors lie a lot (or at the very least bend the truth).1 Brønsted-Lowry Acids and Bases, the hydronium ion molarity in pure water (or any neutral solution) is 1.5 will immediately give a pH value of 6. Now that we know the concentration of hydronium ions in solution, we can use our pH equation to find the pH of water at 50 degrees Celsius. For example, NO−3 NO 3 − is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall −1 charge. Instructor Emily Lockhart View bio. To define the pH scale as a measure of acidity of a solution. Hence a range of 0 to 14 provides sensible (but not absolute) bookends for the scale.Polyatomic ions are molecular ions composed of two or more atoms bonded by covalent bonds and acting as a single unit, but unlike molecules, they have a net charge on them. Author Chase Smith View bio.Worked examples: Calculating [H₃O⁺] and pH.
La réaction entre l’eau et l’acide nitrique forme l’ion oxonium et l’ion nitrate H 2 O + HNO 3 → H 3 O + + NO 3 –.00000031623 M (or 3.3 times 10 to the negative seventh molar.
Strong acid solutions (video)
Identify the Brønsted-Lowry acid and the Brønsted-Lowry base in this chemical equation.
L'ion oxonium
Brønsted acids release one or more of their protons (hydrogen ions), . A coordinate covalent bond also results when an ammonia molecule combines with a hydrogen ion to form an ammonium ion.Si l’espèce chimique est de type AH alors l’équation de réaction a la forme suivante: H 2 O + AH → H 3 O + + A –.Par exemple, l’ammoniac (N H 3) peut réagir avec les ions hydrogène (H +) présents dans l’eau pour produire l’ion ammonium (N H + 4).
Worked examples: Calculating [H₃O⁺] and pH
Un exemple de base d’Arrhenius est l’hydroxyde de sodium très soluble, NaOH .The pH scale is a convenient way to represent the acidity or basicity of a solution. C6H5OH +NH−2 C6H5O− +NH3 C 6 H 5 OH + NH 2 − C 6 H 5 O − + NH 3. This is similar to the simplification of the formula of the hydronium ion, H 3 O + to H +.Thus, both the pH = 7 and pOH = 7 for pure water.1) BF 3 + NH 3 → F 3 B − NH 3. One of these h. L' ion hydronium est le type le plus simple d' ion oxonium . The concentration of H₃O⁺ in a strong acid solution is therefore equal to the initial concentration of the acid. At 25°C, a solution with pH 7 is basic, and a solution with pH = 7 is neutral. As previously noted, temperature matters. To illustrate how this calculator works, let’s consider a solution with a pH of 3. De façon générale, les acides d’Arrhénius sont également des acides de Brønsted-Lowry, étant donné qu'ils peuvent donner des ions hydrogène.00 and pOH values less than 7. Il est produit lorsqu'un acide d'Arrhénius se dissout dans l'eau.Overview
Hydronium Ion or Oxonium
Hydronium is also known as hydroxonium.The hydronium ion is the oxonium cation that forms from the protonation or auto-dissociation of water. Strong acids (such as HCl, HBr, HI, HNO₃, HClO₄, and H₂SO₄) ionize completely in water to produce hydronium ions. Basic solutions are those with hydronium ion molarities less than 1.Acid-base Reactions without Transferring Protons.
Qu'est-ce qu'un ion hydronium
NH3. L'hydronium est également abondant dans le milieu interstellaire et les queues des comètes.In a basic solution, [ OH −] > [ H 3 O +] For aqueous solutions at 25 ∘ C. The contribution of the autoionization of water to [ H 3 .For example, at a pH of zero the hydronium ion concentration is one molar, while at pH 14 the hydroxide ion concentration is one molar.What molecule am I? All acidic aqueous solutions contain protonated water, known commonly as the hydronium ion (H 3 O + ). pH = -log [H 3 O + ], [H 3 O +] = 10 -pH = 10 -5. Hydronium is the .
H+ Concentration from pH
For example, a solution of 0.For example, a coordinate covalent bond occurs when a water molecule combines with a hydrogen ion to form a hydronium ion. See examples of HYDRONIUM ION used in a sentence.Strong acid solutions. Definition: Lewis Acids and Bases. Check molarity formula using this calculator., acts as both an acid or a base), water does not always .87, is incorporated into the anti-log pH Equation, as shown below.1623 × 10-7 M).0 × × 10 −7 M and hydroxide ion molarities greater than 1.
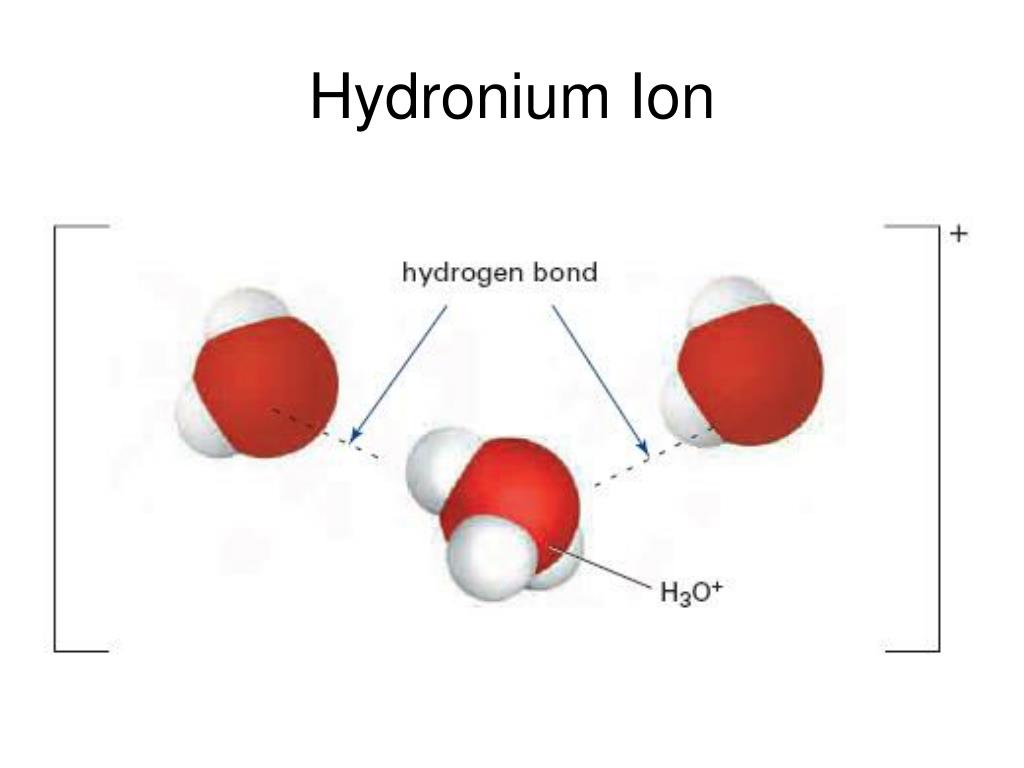
Hydronium ion
001 M, demonstrating the tool’s practicality in converting pH values to H3O+ concentrations . Comme pour de nombreuses espèces en chimie, la .M is the number of moles of the substance per liter of solution. Une base d’Arrhénius est définie comme toute espèce qui augmente la concentration en ions hydroxyde, OH − , en solution aqueuse.Example of H3O+ Concentration for Each pH Calculator.Bases d'Arrhenius. Ion Concentrations in Pure Water. First, we'll walk .Example \(\PageIndex{1}\) Calculate the concentration of hydronium ions that are present in a solution that has a reported pH value of 4. What are the hydronium ion concentration and the hydroxide ion concentration in pure water at 25 °C? This is an example of a neutralization reaction; an acid and a base have reacted to form water. In order to calculate the molar concentration of hydronium ions, H 3 O +1, from a pH value, the given value, 4.
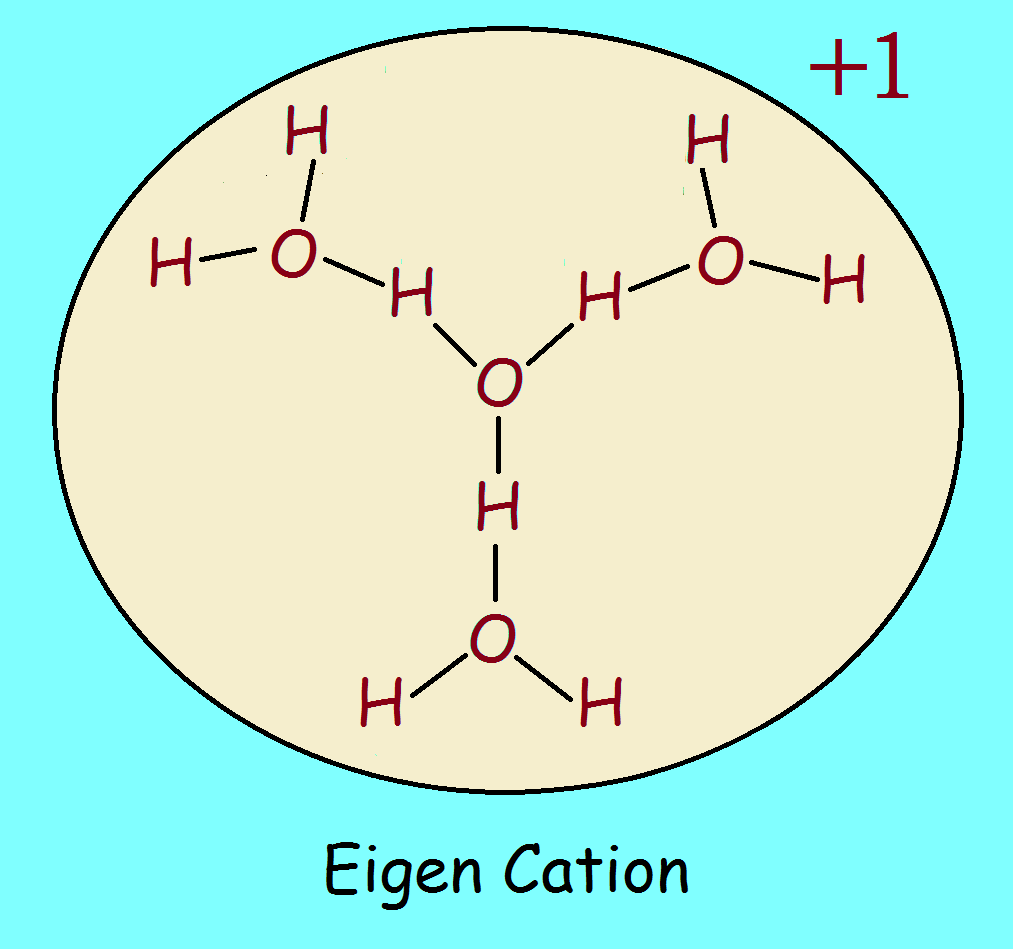
Hydronium ion: Yes or no?
Let's say we made a 2 M aqueous solution of hydrobromic acid, HBr , which is an Arrhenius acid. ions immediately react with water molecules to form hydronium ions, H 3 O +. Note that the more hydrogen ions [H +] the acid provides, the lower .1, the hydronium ion molarity in pure water (or any neutral solution) . For example, at 100 °C, the value for \(K_\ce{w}\) is approximately \(5. The major utility of the Lewis definition is that it extends the concept of acids and bases beyond the realm of proton transfer reactions. The transition happened when I returned to AP Chemistry after a ten-year . A Lewis acid is any .The process is endothermic, and so the extent of ionization and the resulting concentrations of hydronium ion and hydroxide ion increase with temperature.It's pretty much universally known that Chemists are lazy.Learning Objectives. Cependant, il est important de souligner que les acides d’Arrhénius . We can calculate the pH of a solution by taking the negative logarithm of the hydronium ion concentration, or pH = -log [H₃O⁺].
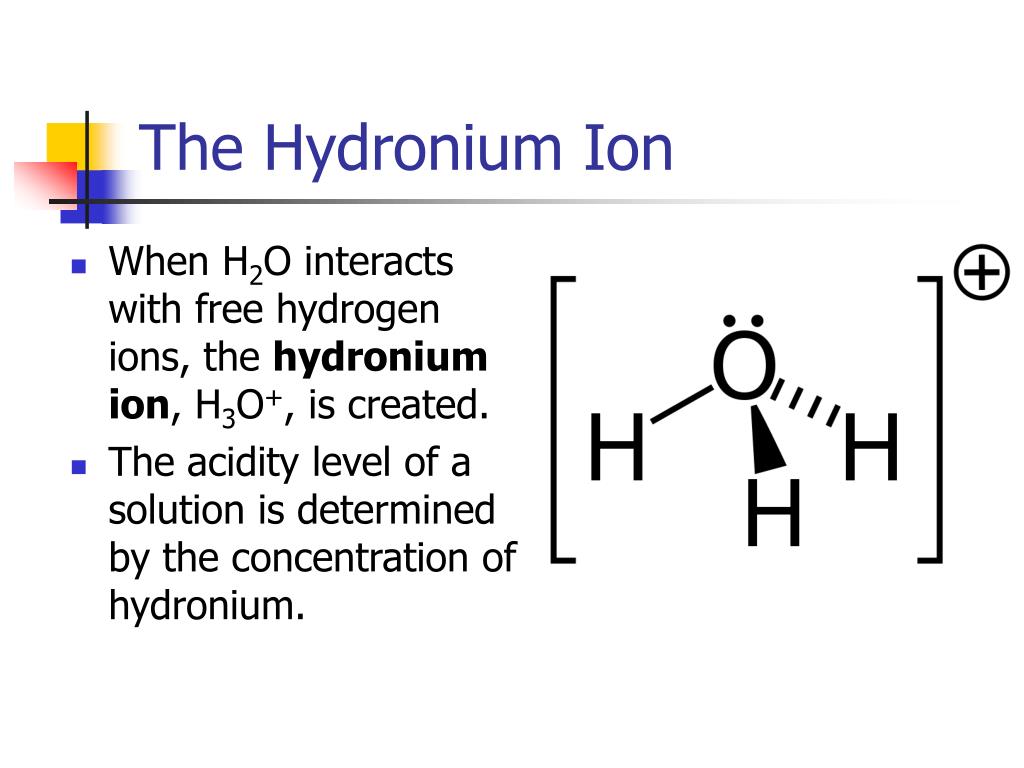
The Hydronium Ion
As was shown in Example 1 in Chapter 14. 1 lists the ion names and ion formulas of the most common polyatomic ions. However, in this case . Which example is a hydronium ion? H3O+ What is formed when an acid dissociates in water, according .If we wish to find the hydronium ion concentration ([H 3 O +]) and the pH of a solution, we need to know both the strength of the acid (or base) and the concentration of the acid (or . Because of its amphoteric nature (i. Which formula is used to determine the pH of a solution? pH=−log[H+] A . , the following relationships are always true: K w = [ H 3 O +] [ OH −] = 10 − 14.As was shown in Example 14. Hydronium is the simplest form of oxonium, which is any ion that contains the trivalent oxygen cation.1 \times 10^{−13}\), roughly 100-times larger than the value at 25 °C. Moreover, Nafion membranes—an . In chemistry, hydronium or the hydronium ion refers to the chemical species H 3 O +.

L'hydronium est également connu sous le nom d'hydroxonium.L'ion hydronium ou hydronium est le nom donné au cation H 3 O + , issu de la protonation de l'eau. Moreover, hydrogen bonds are involved . Google Classroom. This makes sense, since the pH and pOH should sum to 14, as shown in Equation 15. The classic example is the reaction of boron trifluoride with ammonia to form an adduct: BF3 +NH3 → F3B−NH3 (10. Formation de l’ion oxonium par autoprotolyse de l’eau Les ions oxoniums sont naturellement présents .The hydronium ion or hydronium is the name given to the H 3 O + cation, derived from the protonation of water. Which example is a hydronium ion? H3O+ What is formed when an acid dissociates in water, according to the Brønsted-Lowry definition? a conjugate base.1 M H₃O⁺ and has a pH of 1.And since x is equal to the concentration of hydronium ions in solution, the concentration of hydronium ions is 2. Using our formula: [H3O+] = 10^-3. L'hydronium est la forme la plus simple .For example, [H +] = 10-6.Vue d’ensemble
Hydronium
Does that mean we have 2 M of H + ions in our .1 M HNO₃ contains 0. As discussed earlier, hydronium and hydroxide ions .In the autoionization of water, a proton is transferred from one water molecule to another to produce a hydronium ion (H₃O⁺) and a hydroxide ion (OH⁻).
Express hydronium and hydroxide ion concentrations on the pH and pOH scales.When we talk about acids in chemistry we’ll often see H+ and H3O+ written. Perform calculations relating pH and pOH.For example, hydronium ion-based acid pretreatment is utilized to break down biomass into sugars for biofuel production.Which example is a Brønsted-Lowry base but not an Arrhenius base? NH3. in aqueous solution.0 × 10 −7M at 25 °C.We frequently see the formula of this ion simply as “Al 3+ (aq)”, without explicitly noting the six water molecules that are the closest ones to the aluminum ion and just describing the ion as being solvated in water (hydrated).You get the same result if the [H +] ion concentration is written as 0. Both of these equations are shown here.
Ion hydronium — Wikipédia
The hydronium ion is the simplest type of oxonium . Since the autoionization constant K w .