Igg4 receptor binding
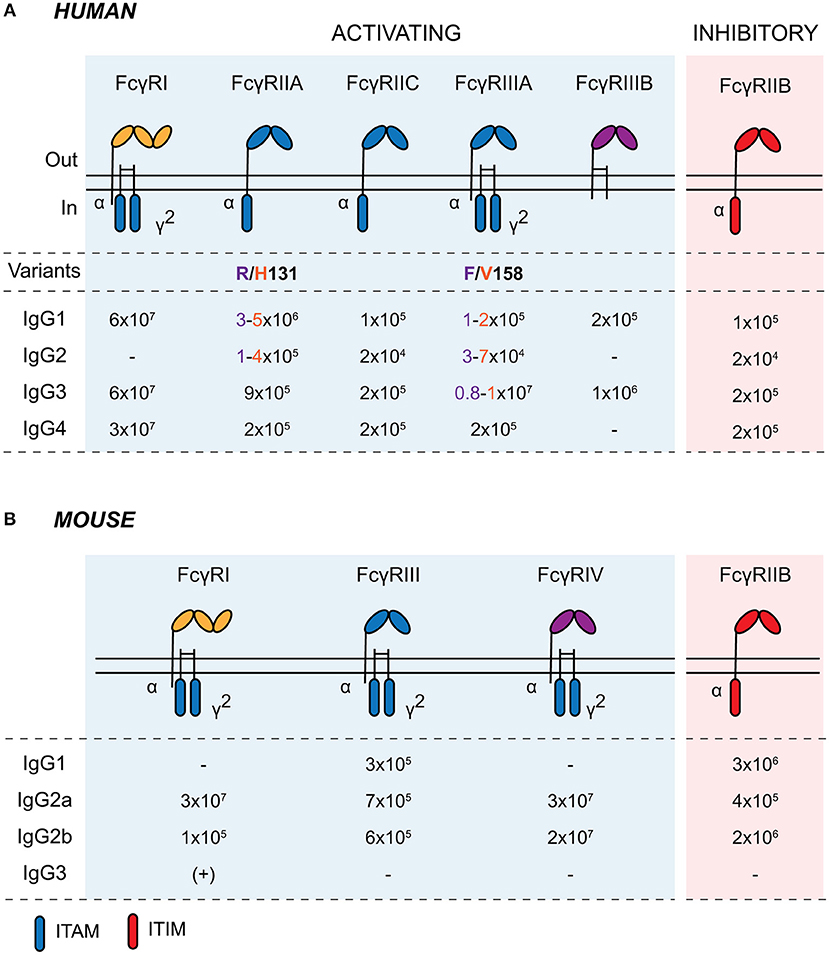
2015 Apr;23 (4):757-68. Here, we report a novel assay format to . For individual receptor subtypes, IgG1 could bind to all three receptor subtypes, that is, FcγRⅠ (CD64) > FcγRⅡ (CD32) > FcγRⅢ (CD16).
The unique properties of IgG4 and its roles in health and disease
Insulin receptor binding motif tagged with IgG4 Fc (Yiminsu) works as an insulin sensitizer to activate Akt signaling in hepatocytes.Among the four subclasses of human IgG (IgG1, IgG2, IgG3, IgG4), which differ in their constant regions, particularly in their hinges and CH2 domains, IgG1 has . Therefore, IgG1σ and IgG2σ are among the most effective mutations for knocking out Fc effector function.We found that IgG4 could compete with IgG1 in binding to the Fc receptor of monocytes (U937).IgG4-related disease ( IgG4-RD ), formerly known as IgG4-related systemic disease, is a chronic inflammatory condition characterized by tissue infiltration with lymphocytes and . 4-1BB mutations I64R and V71R showed reduced .Finally, Ig transport is made by FcR-like molecules such as the poly-Ig receptor or an MHC-like receptor found on neonatal intestine.IgG4 is the least abundant subclass of IgG in human serum and has unique functional features.The receptor-binding interface was confirmed by ELISA.
The binding kinetics and affinities of IgG-FcγR interactions are hence important parameters for understanding FcγR-mediated immune functions. View PDF View article View in Scopus Google Scholar [21] T. This leads to reduced densities of the .The affinity and binding specificity of the Fc domain for different FcγRs are determined by differences in the primary amino acid sequence of the IgG subclasses .IgG4 contains unique structural features in the hinge, C H 2 and C H 3 domains, that are thought to be responsible for its structural properties, binding characteristics and reduced effector function, compared to other subclasses. 6 However, the affinity of specific glycoforms of IgG4 for FcγIIIA is unknown. IgG4 is largely unable to activate antibody-dependent immune effector .Instead, IgG4 relies on Fc-independent mechanisms, which is a block of protein-protein interaction with different consequences (Fig. Besides variation in terms of half-life, antigen . G921 is effective in a concentration-dependent manner in a focus reduction neutralization assay with EC 50 =16.Percentages were calculated from IgG4-binding cells and the sum of cells of all four IgG subclasses. Antigen-induced allosteric changes in a human IgG1 Fc increase low-affinity fcgamma receptor binding.
FcγR Binding and ADCC Activity of Human IgG Allotypes
Characterization and screening of IgG binding to the neonatal Fc receptor
Human IgGs bound to mouse FcγR with remarkably similar binding strengths as we know from binding to human ortholog receptors, with relative affinities IgG3>IgG1>IgG4>IgG2 and FcγRI>>FcγRIV>FcγRIII>FcγRIIb.The neonatal Fc receptor (FcRn) is expressed by cells of epithelial, endothelial and myeloid lineages and performs multiple roles in adaptive immunity.
IgG4 is the least abundant subclass of IgG in normal human serum, but elevated IgG4 levels are triggered in response to a chronic antigenic stimulus and .We found that (1) IgG1 and IgG3 bind to all hFcγRs; (2) IgG2 bind not only to FcγRIIA H131, but also, with a lower affinity, to FcγRIIA R131 and FcγRIIIA V158; (3) .We first show that the de novo structures of IgG1 and IgG4 predicted using AlphaFold, with no interactions between the fragment crystallizable (Fc) domain and the .

Thus, to validate the safety of mRNA-based vaccination, it is crucial to unravel the consequences of repetitive administration of mRNA-based COVID-19 vaccinations on antigen-specific IgG subclass Ab response in detail and determine whether there exists any causal relationship between breakthrough infection and anti-receptor .The results we obtained with mouse–human chimeric antibodies came as a surprise: in this situation IgG4 and IgG1 antibodies cross-linked antigen equally well. 2 B): direct blocking of receptor or enzyme function, as seen in thrombotic thrombocytopenic purpura [23] or AChR-MG, stimulation of the receptor by binding as seen with anti-TSHR antibodies in Grave's .FcγR binding characterization is one of the critical attributes during the development of therapeutic antibodies.Moving the Fab region to other IgG subclasses, such as IgG2 or IgG4, .G921 demonstrates ACE2 enzymatic activity comparable to positive control and binding to the neonatal Fc receptor (FcRn) without binding to low affinity Fc-gamma receptors (FcγRs).

In vivo pharmacokinetic enhancement of monomeric Fc and
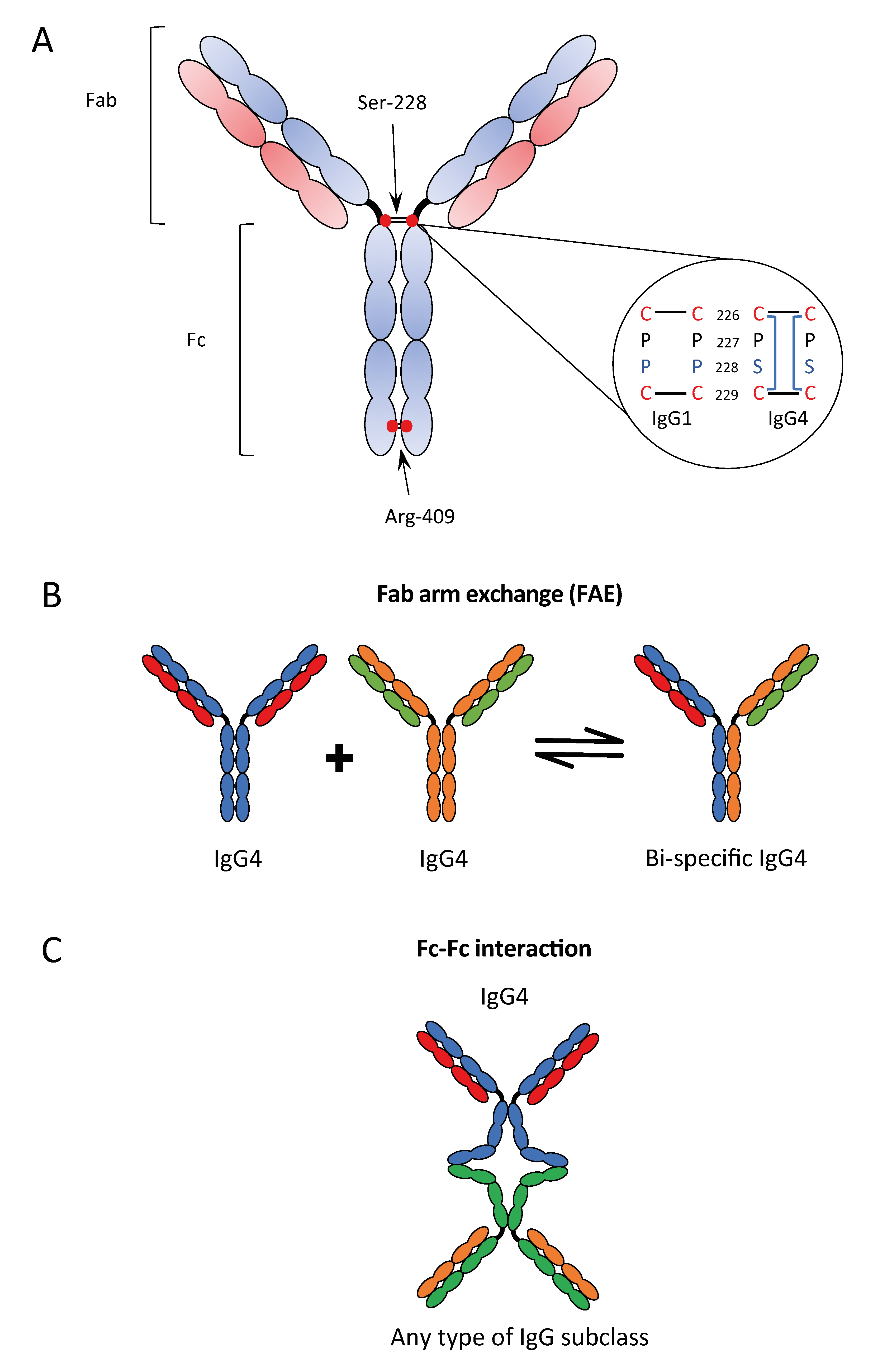
The potential binding of murine IgG1 or human IgG4 to an antigen in close vicinity to the C1q-binding IgG subclasses might interfere with Fc:Fc contact formation, potentially leading to reduced hexamerization, C1q binding, and complement activation (23, 25).An immune evasion mechanism with IgG4 playing an essential role in cancer and implication for immunotherapy | Journal for ImmunoTherapy of Cancer. Structure, 28 (5) (2020), pp.Recent findings: IgG4 antibodies have unique structural and functional properties and undergo 'half-antibody exchange' in vivo, resulting in recombined antibodies composed . 6 High-affinity FcγRs (K A ∼ 10 7-10 8 M −1 for IgG), but not low-affinity FcγRs (K A ∼ 10 5-10 6 M −1 for IgG), are defined by their ability to bind IgG as monomers.Human IgG4, normally the least abundant of the four subclasses of IgG in serum, displays a number of unique biological properties. It binds human IgG1, IgG3, and IgG4 with a high affinity and has no affinity for IgG2. In line with the flow cytometric data, 16% of the sorted spike-specific . 2015 Aug 3;14 . It can undergo heavy-chain .1038/s41577-023-00871-z CARs frequently incorporate a spacer/linker region based on the constant region of either IgG1 or IgG4 to connect extracellular ligand-binding with intracellular signaling domains. Moreover, IgG4 can also bind to FcγRIIb, leading to . both IgG2 and IgG4 are known for limited FcγR engagement and reduced antibody dependent .Previous studies reported the affinity between FcγRIIIA and human serum-derived IgG4 with an equilibrium dissociation constant of 4-5 μM, where IgG4 potentially contained a mixture of various glycoforms.Binding of pathogen-bound immunoglobulin G (IgG) to cell surface Fc γ receptors (FcγRs) triggers a wide variety of effector functions. to label spike-binding B cells and receptor binding domain (RBD)–binding B cells and enriched for spike-binding IgG + B cells by flow cytometry before scRNA-seq.
The role of IgG Fc receptors in antibody-dependent enhancement
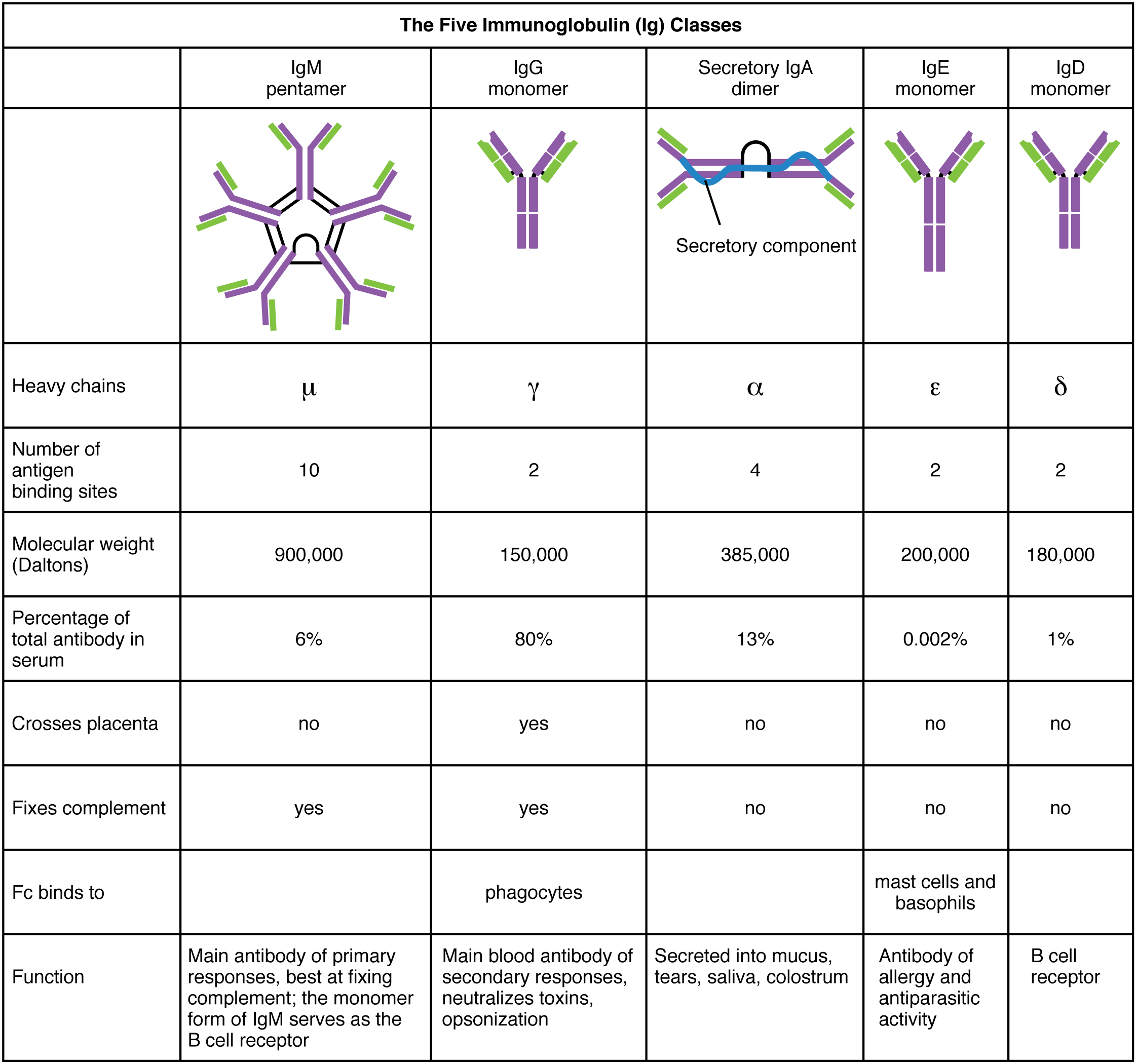
IgG Production by B Cells and Class Switching.
Anti-spike IgG4 rises from obscurity
gen receptor (CAR)–expressing T cells partly depends on optimal CAR design.
Antigen improves binding of IgGs to FcγRs in SPR analysis
The second and more critical pathway is the direct inhibition of IgE-mediated mast cell activation ( Figure 2 ).Recombinant human IgG antibodies (hIgGs) completely devoid of binding to Fcγ receptors (FcγRs) and complement protein C1q, and thus with abolished immune . The neonatal Fc receptor (FcRn) protects immunoglobulin G (IgG) from degradation and increases the serum half-life of IgG, thereby contributing to a higher concentration of IgG in the serum.Demonstrate IgG2 binding to FcgRI and FcgRIIIa-158F in the presence of antigen which has never been reported previously . Characterizing the FcRn/IgG interaction is fundamental to designing therapeutic antibodies because IgGs with moderately increased binding affinities .hFcγRI is the only high-affinity IgG receptor in humans. We further found that the binding force of IgG1 was about twice as strong as that of IgG4. Both types of FcRs bind IgG when . Here, we evaluated the potential for the IgG4-Fc linker to result inThe salty oligopeptides from Stropharia rugosoannulata have been proven to be potential ACE inhibitors. L235E, and P331S), which lacks immune receptor binding 97 and “YTE” (M252Y, S254T, and T256E), which has an extended . This suggests human IgG subclasses to have .Here, we report binding affinities of all mouse and human IgG subclasses to mouse FcγR.Second, binding of Fc to the surface of the receptor involves an asymmetric surface, a property that is common to all Fcγ receptors.

Proliferating B cells undergo class switch .
The unique properties of IgG4 and its roles in health and disease
Efficient steric interference may be dependent on antigen density and epitope . Considering that the microheterogeneity of Asn 297 glycans .Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy.Furthermore, the CH2 and CH3 domains in the Fc fragments of immunoglobulins are responsible for their binding to the Fc receptor, which triggers transcytosis. A remarkable property of most FcR is the fact that they are released in cell supernatants and circulate in biological fluids as immunoglobulin binding factors (IBF) generated either by cleavage at the cell .
FcRn engagement of . IgG4, the least represented human IgG subclass in serum, is an intriguing antibody with unique biological properties, such as the ability to undergo Fab-arm exchange and limit immune complex formation. We have measured the kinetic rates and equilibrium dissociation constants of .The interaction of albumin with neonatal Fc receptor (FcRn) is responsible for its long half-life (~21–28 days) and high concentration (35–55 g/L) in the circulation 58, 67. In contrast, IgG4 . present a structure-guided approach to engineer a monovalent form of the fragment crystallizable (Fc) region of an IgG4 antibody to adapt multiple versions of half-life extension .

The in vivo roles of IgG receptors have been addressed using specific FcR knockout mice or in mice expressing a single FcR, and have demonstrated a .We produced all 27 known IgG allotypes and assessed FcγRIIIa binding and ADCC activity.In case of very low affinity (hIgG2 and hIgG4 binding to FcγRIIb and FcγRIII, and IgG4 binding to FcγRIV), fits were performed by fixing R max values obtained using IgG1/3 with the same receptor densities, as the theoretical maximal number of available IgG-binding places on a sensor surface is independent of affinity and therefore subclass.Auteur : Xin Chen, Xiaomin Song, Kang Li, Tong Zhang