Residual dna analysis

The ddPCR CHO Residual DNA Quantification Kit can reliably detect as little as 1 fg of DNA with a limit of quantification (LOQ) of ≤15 fg per 20 µl reaction and a linear range of 3 . Levels of host cell DNA needs to be monitored during process development and validation.Temps de Lecture Estimé: 6 minThe current method for residual DNA quantification in rAAV was adapted from protein programs and required sample digestion by proteinase prior to qPCR analysis.Forensic DNA analysis - Wikipedia. Morgan, Gan Wang, Ned M.Auteur : Xing Wang, Donna M. Although many labs opt for in-house methods to screen for residual DNA, these can be costly, time .Recent advances in circulating tumor DNA (ctDNA) analysis have shown promise in monitoring patients with nonmetastatic cancer, but these have primarily focused on recurrence monitoring and have limited accuracy for residual disease detection during treatment (6–9). Analytical methods to characterize these . The report may be the best of what is a .Residual DNA analysis in biologics development: Review of measurement and quantitation technologies and future directions. Digital PCR (dPCR) technology is widely applied in DNA quantitative analysis due to high specificity, sensitivity, absolute quantification, etc. The Applied Biosystems® PrepSEQ® Residual DNA Sample Preparation Kit is optimized for highly efficient DNA recovery from complex mixtures of proteins, buffers, and salts which are typical of samples that need to be quantitated for residual host cell line DNA. The LOD and LOQ of 200 bp DNA marker were 2. Standard solutions: Dilute the DNA standard stock solution to obtain five or more suitable . Monitoring the production of antibodies to detect harmful . Creative Proteomics provides a sensitive and accurate analysis to quantitate a broad .With the help of residual DNA analysis you can secure that your products fulfill the regulations and guidelines of the EFSA, FDA, WHO or EMA. In order to assess the range of the direct resDNA qPCR method and to develop an easily workable . Current methods. Rufen Sie uns an +49 8092 8289-0 ** The residual DNA in the sample was analyzed according to the standard curve. First, the application of naked oncogenic DNA to mouse skin .Residual DNA – Analyse der DNA und RNA Restmengen von Wirtszellen bei Biopharmazeutika und anderen biotechnologischen Produkten.3 Bulk Lot Release Testing 7.1 Introduction 8. DQ alpha testing.64 pg/ml, respectively. Analysis of ctDNA can . • Based on Cygnus’ proprietary residual host cell DNA extraction methodValidation of residual host cell DNA analysis 6 Contamination control and assay validity 6 Conclusion 7.
However, low levels of residual or plasmid DNA can often remain after purification, affecting the safety and efficacy of final products.
Characterization of DNA Impurities in Cell Therapies
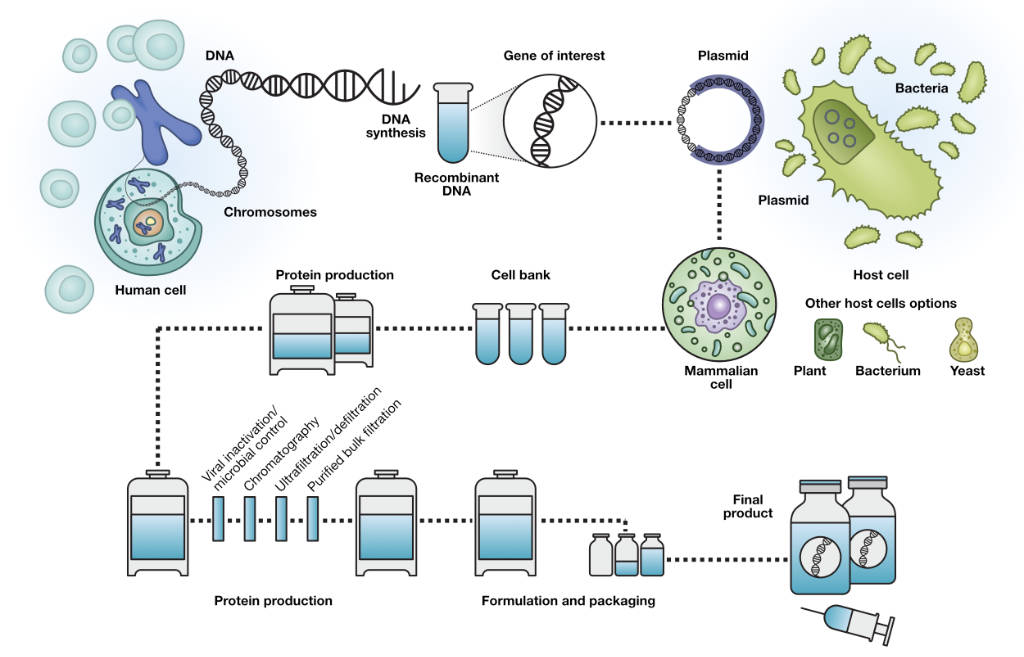
Innovator Insight.Residual DNA (rDNA) is defined as the sum total of deoxyribonucleic acid (DNA) and fragments present in biological samples derived from recombinant host cells during .
Residual DNA Size Analysis Kit-Human cell
The Residual DNA Size Analysis Kit eliminates the need for expensive and time-consuming manual DNA extraction procedures.Validation of these DNA analysis methods usually includes the evaluation of the reproducibility, cross-reactivity, DNA template concentration, precision, environmental . Published: 12 August 2022. It combines a sample preparation method that enables quantitative DNA recovery with high precision from a variety of sample matrices, and specific qPCR-based assays for the host cell line . Currently, real-time quantitative PCR (qPCR) based methods are widely employed .Host residual DNA (hrDNA) is an impurity and needs to be monitored in the purified drug to ensure purity and safety.The Residual DNA Size Analysis Kit is user-friendly and easy to use, making it accessible to researchers with varying levels of expertise.Using DNA standard substance of known concentration and according to the above relationship, constructing a standard curve and quantitatively analyzing the specific template, the residual amount of exogenous DNA in the test sample can be determined.
Call +49 8092 8289-0 ** Avance Biosciences™ also offers Southern blot service to .Global Host Cell Residual DNA Detection Market Report 2023 comes with the extensive industry analysis of development components, patterns, flows and sizes. Electrophoresis.The experiment shown in Table 1 demonstrated that digestion of 50–100 μg IgG1-1 drug substance with KAPA protease followed by heat denaturation and qPCR, could be a good residual DNA method with about 80% spike recovery. coli Residual DNA Quantification Kits enable highly sensitive and precise detection and quantification of host cell DNA without a standard curve.The 200 bp DNA marker had a good linearity between 50 and 1000 pg/ml.Residual host cell DNA is an impurity that has been associated with the risk of infection and oncogenicity.
Residual DNA Size Analysis Kit-Vero (RDQK-08)
2024 Feb 1;84(3):468-478.
Establishing Acceptable Limits of Residual DNA
In addition, this method was successfully used to analyze the size distribution analysis of residual HCD fragments in lentivirus products with different production processes.With our expertise and extensive experience in the “Gold-standard” real-time qPCR assay development and validation, Avance Biosciences™ offers customized residual DNA analysis services to determine the residual host cell DNA in biological products derived from cell substrates.
Residual DNA: DNA & RNA Restmengen-Analyse von Wirtszellen
Residual DNA analysis in biologics development
Capillary gel electrophoresis with laser‐induced fluorescence detector with CGE‐LIF method was successfully used to analyze the size distribution analysis of .
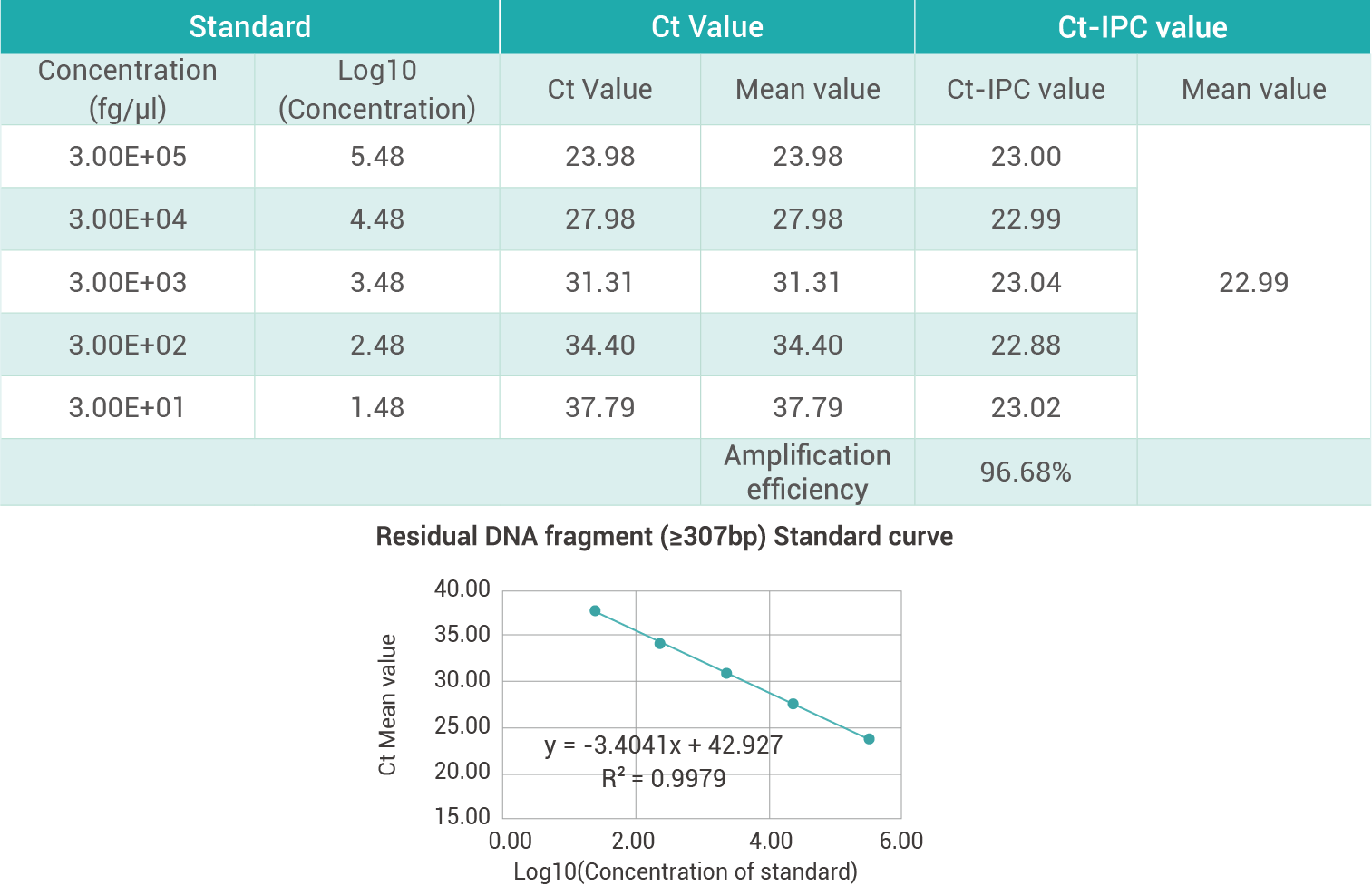
Thus, it needs to be monitored for quality control. Quantitative and consistent recovery of DNA can be obtained from .
PF 42(5)
Size distribution analysis of residual host cell DNA fragments in lentivirus by CGE‐LIF.The residual DNA in biological products is considered a risk factor due to its potential tumorigenicity, infectivity, and immunogenicity, requiring detection and removal .Specifically, two important host cell impurities of focus within biopharmaceuticals are residual DNA and protein.Certal Residual DNA Detection Kits enable sensitive and reliable detection and quantification of residual genomic DNA (gDNA), which is essential for the production of safe and reliable biopharmaceuticals (see figure Highly sensitive and reliable detection ).Residual DNA (rDNA) is comprised of deoxyribonucleic acid (DNA) fragments and longer length molecules originating from the host organism that may be present in samples from recombinant biological processes.Residual DNA: Analysis of residual DNA and RNA of host cells within biopharmaceuticals and other biotechnological products.In our analysis we compared highly diverse MRD-test targets (specific cancer markers like BCR::ABL1, clonal markers such as IG/TCR rearrangements, aberrant cell . During the production of cell and gene therapy products, residual host cell DNA (HCD) could cause safety risks of the biological products, and the longer .
DNA Analysis
ddPCR Residual DNA Quantification Kits
Your review can help your fellow researchers make informed purchasing decisions.Quantitation of residual DNA in final product biopharmaceuticals using the Threshold Total DNA assay system.Standards Preparation Mozier
Residual DNA
extraction of DNA to remove PCR interfering components, residual host cell DNA DNA concentrate for standards, a qualified cell-line specific primer set, and a PCR assay plate with optical seal.
Biotherapeutics must go through rigorous purification processes during their development and manufacturing.Residual DNA (rDNA) is comprised of deoxyribonucleic acid (DNA) fragments and longer length molecules originating from the host organism that may be present in samples from recombinant biological .
PrepSEQ™ Residual DNA Sample Preparation Kit
Certal Residual DNA Detection Kits能够灵敏、可靠的定量检测残留的基因组DNA(gDNA),这对生产安全和可靠的生物制品至关重要(参见 Highly sensitive and reliable detection )。 检测残留的DNA需要最小限度的检测和高线性范围,以确保可靠的检测,即使残留的gDNA的水平很低(参见 Low limit of detection )。 The diagram below represents the results of a procedure . Biological products can contain residual DNA from host cell substrates. DNA samples were collected from four organisms: A, B, C, and D.

1 Introduction 10.Serial Circulating Tumor DNA Analysis with a Tumor-Naïve Next-Generation Sequencing Panel Detects Minimal Residual Disease and Predicts Outcome in Ovarian Cancer Cancer Res . Cell & Gene Therapy Insights 2022; 8(7), 837–840 DOI: 10. Data support is relatively lacking in deciphering the dPCR technology . 3 The expression of biological products using recombinant DNA technology has enabled the use of peptides and proteins for therapeutic use. Google Classroom.Simplifying residual DNA analysis in viral vector production: focus on E1A .
Host Cell Residual DNA Analysis
Aims of the analysis.DNA probe solution: Dilute DNA stock probe to 2.The Certal CHO Detection Kit detects residual gDNA from Chinese Hamster Ovary (CHO) .resDNASEQ System for Residual DNA Testing.Residual host-cell DNA is a process impurity in recombinant biotherapeutic products.
Personalized circulating tumor DNA analysis to detect residual
In particular, detection of ctDNA after completion of NAT has been .

Write a review of your use of Biogene products and services in your research. The report also calculates present and past market values to forecast potential market management through the forecast period between 2023-2029.Circulating tumor DNA (ctDNA) consists of DNA fragments released from cancer cells into the blood circulation with quick clearance. Residual DNA can be measured to 1 part per billion when using this kit. Assay validation and determination of detection limit | Molecular . Product Name * . 8 Residual DNA Testing Market, By Application 8. Wentao Wang Tie Gao +4 authors Hongxu Chen.Residual DNA impurities are a critical quality attribute (CQA) due to their potential impact on product quality and safety. In this summury, Srinath Kashi Ranganath summarises how residual DNA analysis of the E1A fragment can be performed for therapeutic-grade AAV .










