Tisagenlecleucel fda approval
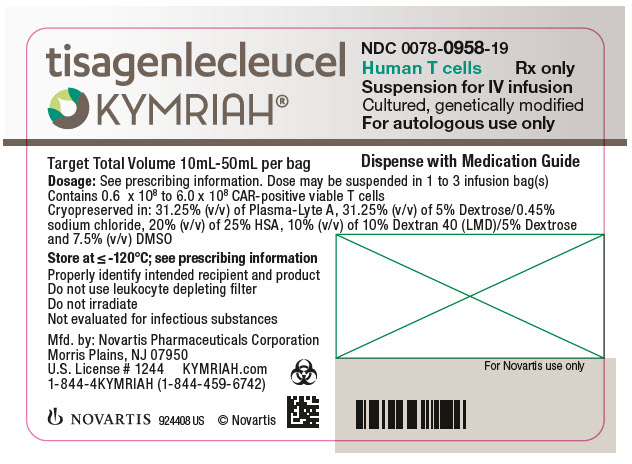
This is a type of medicine that works by delivering genes into the body.FDA approval for Tisagenlecleucel (CTL019, Kymriah, Novartis, . ¹This use is approved under FDA’s Accelerated Approval Program.The FDA has granted accelerated approval to tisagenlecleucel for the treatment of adult patients with relapsed or refractory follicular lymphoma following 2 or more lines of systemic therapy.Basel, August 30, 2017 - Novartis announced today that the US Food and Drug Administration (FDA) has approved Kymriah (TM) (tisagenlecleucel) suspension for .Auteur : Maura C.Kymriah is a type of advanced therapy medicine called a ‘gene therapy product’.
FDA approves Novartis Kymriah® CAR-T cell therapy for adult
Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for therapeutic purposes) Drug Approval Reports by Month. It showed particularly high efficacy in R/R follicular lymphoma (FL) with a manageable toxicity profile. O'Leary, Xiaobin Lu, Ying Huang, Xue Lin, Iftekhar Mahmood, Donna Przepiorka, Denise Gavin,. The approval is based on findings from the Phase II ELARA trial, which evaluated 90 patients for efficacy with a median follow-up of 17 months. On August 30, 2017, the FDA . Proper Name: tisagenlecleucel.On May 27, 2022, the Food and Drug Administration granted accelerated approval to tisagenlecleucel (Kymriah, Novartis Pharmaceuticals Corporation) for adult patients .
Tisagenlecleucel T
Tisagenlecleucel is an autologous anti-CD19 chimeric antigen receptor-T cell therapy with clinically meaningful .) a CD19-directed genetically modified . It is the largest study examining a CAR-T therapy in DLBCL, enrolling patients from 27 sites in 10 countries across the US, Canada, Australia, Japan, Austria, .Basel, May 28, 2022 — Novartis today announced the US Food and Drug Administration (FDA) has granted accelerated approval for Kymriah ® (tisagenlecleucel) for the treatment of adult patients . Generic name: tisagenlecleucel. Manufacturer: Novartis Pharmaceuticals Corporation.On May 1, 2018, the Food and Drug Administration approved tisagenlecleucel (KYMRIAH, Novartis Pharmaceuticals Corp.If you would like more information, the FDA-approved product labeling for KYMRIAH can be found at www.Tisagenlecleucel was the first gene therapy to receive approval from the FDA for any indication.
FDA Approval Summary: Tisagenlecleucel for Treatment of
Kymriah (tisagenlecleucel) Tecartus (brexucabtagene autoleucel) .Chimeric antigen receptor (CAR) T-cell therapy has become a powerful treatment option in B-cell and plasma cell malignancies, and many patients have . During the study, prolonged durable response to treatment was demonstrated, with . Slide 18: Difference Between CAR T-Cells This slide here hopefully is visible.EAST HANOVER, N.Introduction: Tisagenlecleucel (tisa-cel) is an anti CD19 CAR-T therapy that has demonstrated clinical activity in R/R large B-cell lymphoma and R/R B-cell acute lymphoblastic leukemia.Tisagenlecleucel is FDA-approved in two other indications: pediatric and young adult B-cell acute lymphoblastic leukemia and adult R/R diffuse large B-cell lymphoma.Tisagenlecleucel is an anti-CD19 chimeric antigen receptor T-cell therapy approved for diffuse large B-cell lymphoma after at least two treatment lines.The FDA has granted accelerated approval to tisagenlecleucel (Kymriah; tisa-cel) for the treatment of adult patients with relapsed/refractory (R/R) follicular lymphoma after receiving 2 or more lines of systemic therapy, according to Novartis.this is also a more recent FDA approval and a more recent therapy that’s become available.FDA Approved: Yes (First approved August 30, 2017) Brand name: Kymriah. 30, 2017 /PRNewswire/ -- Novartis announced today that the US Food and Drug Administration (FDA) has approved Kymriah ™ (tisagenlecleucel) .Active Ingredient:Tisagenlecleucel License Number:MOHW-BI 001176 Applicant:台灣諾華股份有限公司 Approval Date:2021.The FDA gave the green light to Novartis's tisagenlecleucel for the treatment of acute lymphoblastic leukaemia (ALL), marking a historic approval for a first-in-modality chimeric antigen receptor .The emergence of cell and gene therapies has dramatically changed the treatment paradigm in oncology and other therapeutic areas. This approval follows .Approval was based on ZUMA-7, a randomized, open-label, multicenter trial in adult patients with primary refractory LBCL or relapse within 12 months following completion of first-line therapy .Tisagenlecleucel is an autologous chimeric antigen receptor (CAR) T-cell therapy that targets the CD19 protein common to B cells.
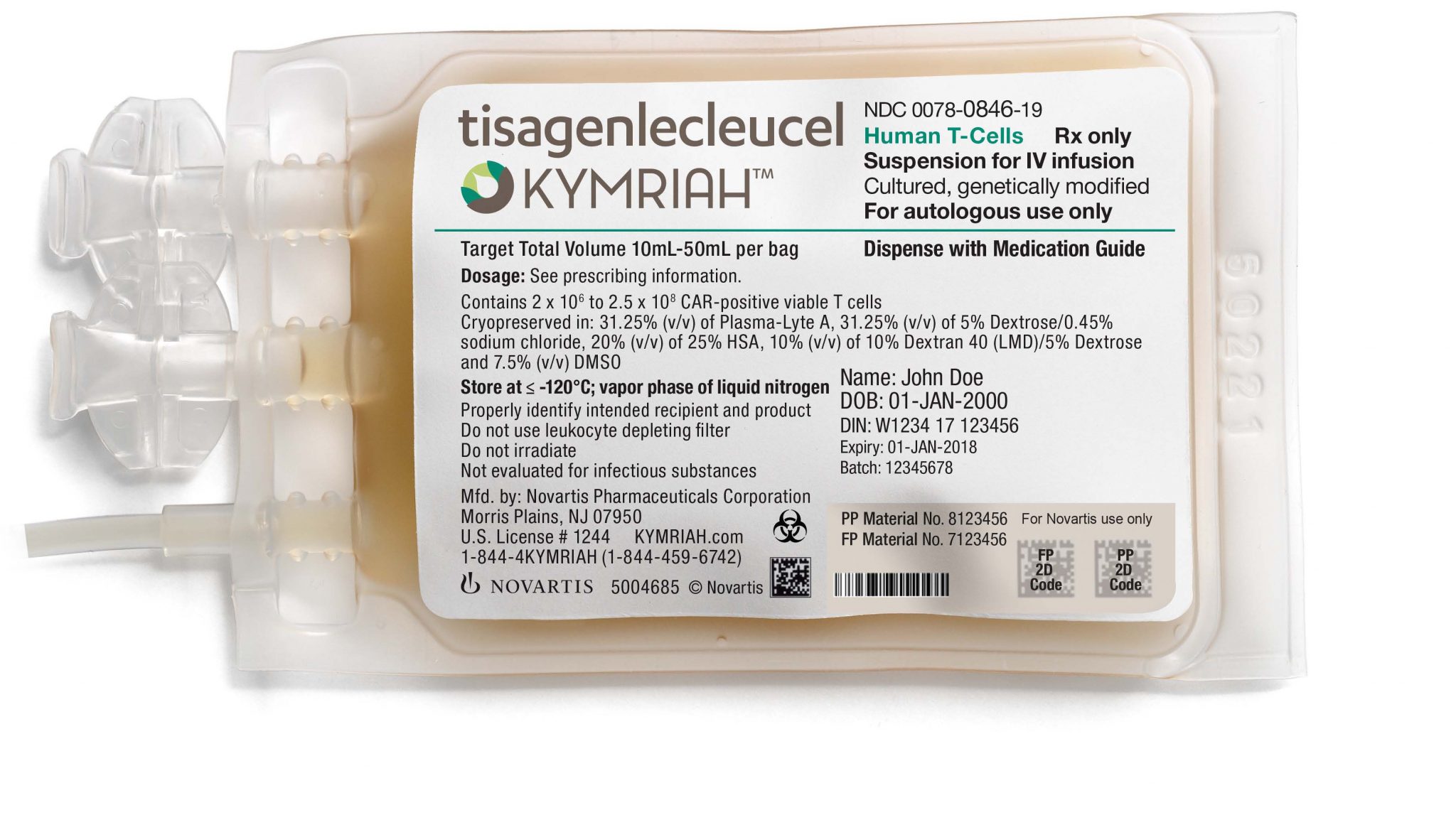
Tisagenlecleucel is an autologous genetically modified immunocellular therapy indicated for the treatment of pediatric and young adult patients 3 to 25 years of age with
DailyMed
Aug 30, 2017 - First-in-class therapy showed an 83% (52/63) overall remission rate in this patient population with limited treatment .
FDA Requires Boxed Warning for T cell Malignancies
This landmark step brought CAR T-cell therapy to the commercial space and heralded a new era in managing refractory B-cell malignancies and FDA oversight of gene-modified therapies.

FDA concluded that changes to the Boxed Warning are warranted to highlight the serious risk .Also on May 27, 2022, the FDA granted accelerated approval to tisagenlecleucel (brand name Kymriah) for adult patients with relapsed or refractory follicular lymphoma after two or more lines of .orgRecommandé pour vous en fonction de ce qui est populaire • Avis
KYMRIAH
The median time from leukapheresis to tisagenlecleucel infusion in the tisagenlecleucel group was 52 days (range, 31 to 135) in the overall population, 41 days (range, 31 to 91) in U. However, its peculiar and severe toxicities must be improved in future trials. Tradename: KYMRIAH. Approval was based on an 81% complete response rate in the registrational trial (ELIANA), with long term follow-up data .Optimal structure, T-cells selection and enrichment, doses, and lymphodepleting regimen remain relevant and hot topics in the field of cellular therapy.En mai 2018, la FDA a en outre approuvé tisagenlecleucel pour le traitement des adultes atteints de lymphome diffus à grandes cellules B récidivant ou réfractaire (LDGC . In spite of those questions, tisagenlecleucel has proven its efficacy leading to its approval by the FDA.The FDA approves tisagenlecleucel (Novartis) for the treatment of patients with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later .The FDA approved Kymriah (tisagenlecleucel) for certain pediatric and young adult patients with a form of acute lymphoblastic leukemia (ALL).Basel, May 1, 2018 - Novartis today announced the US Food and Drug Administration (FDA) has approved Kymriah ® (tisagenlecleucel) suspension for intravenous infusion . 1 The basis of the approval comes from data concluded in the phase 2 ELARA trial (NCT03568461), examining .orgTisagenlecleucel in Adult Relapsed or Refractory Diffuse .Novartis receives first ever FDA approval for a CAR-T cell therapy, Kymriah™ (tisagenlecleucel, CTL019), for children and young adults with B-cell ALL that is refractory or has relapsed at least twice.FDA Approval of Tisagenlecleucel: Promise and Complexities of a $475 000 Cancer Drug.Second-Line Tisagenlecleucel or Standard Care in . Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA Dosage form: Suspension for Intravenous Infusion. The FDA’s approval was granted based on the results of the phase II ELARA trial. Indication: KYMRIAH is a CD19 . This approval has important .KYMRIAH (tisagenlecleucel) is a CD19-directed genetically modified autologous T cell immunotherapy comprised of autologous T cells that are genetically modified using a lentiviral vector to encode an anti-CD19 chimeric antigen receptor (CAR).com, or call 1-844-NVS-CART (1-844-687-2278). Here we review the pre-clinical and clinical development of what .In August 2017, the FDA took historic action in granting the first approval of gene therapy to tisagenlecleucel.
KYMRIAH® (tisagenlecleucel)
Kymriah® (tisagenlecleucel), a CD19-directed CAR-T, was the first cell-based gene therapy approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of relapsed/refractory (r/r) pediatric and young adult ALL, following a unanimous recommendation for approval by the FDA’s Oncology Drug Advisory . Kymriah® (tisagenlecleucel), a CD19-directed genetically modified autologous T-cell immunotherapy, is currently approved in major markets for the treatment of relapsed/refractory (r/r) pediatric and .Tisagenlecleucel is an autologous anti-CD19 chimeric antigen receptor-T cell therapy with clinically meaningful outcomes demonstrated in patients with relapsed/refractory (r/r) B-cell lymphoma. The blood cancers that Kymriah is used to treat are rare, and Kymriah was designated an ‘ orphan medicine ’ (a medicine used in rare diseases) for B-cell ALL on 29 April 2014, DLBCL on .Tisagenlecleucel (Kymriah; Novartis Pharmaceuticals) is a CD19-directed genetically modified autologous T-cell immunotherapy.The FDA approval of Kymriah ® in adult patients with r/r DLBCL is based on the pivotal, single-arm, open-label, multicentre phase II JULIET clinical trial (NCT02445248).The FDA gave the green light to Novartis's tisagenlecleucel for the treatment of acute lymphoblastic leukaemia (ALL), marking a historic approval for a first .
Tisagenlecleucel (Kymriah) Approved to Treat Some Lymphomas
This agent is the .
Kymriah (tisagenlecleucel) FDA Approval History
On August 30, 2017, the FDA approved tisagenlecleucel for treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) .Tisagenlecleucel is only available as part of a special program called Kymriah REMS (Risk Evaluation and Mitigation Strategies).
Tisagenlecleucel
FDA Expends Approval for CAR-T Therapy, Tisagenlecleucel
On 30 th August 2017, tisagenlecleucel became the first chimeric antigen receptor (CAR)-T-cell therapy to be approved by the FDA.23 Indication: Kymriah是一種經過基因修飾的自體免疫細胞療法,適用於治療: (1) 患有難治型、移植後復發、第二次或二次以上復發之B細胞急性淋 巴性白血病(ALL)的25歲以下兒童和年輕成人病人。 (2 .Tisagenlecleucel, the sole CAR approved for pediatrics, is a CD19-specific autologous T cell product that was FDA approved for patients ≤25 years with refractory B-ALL or B-ALL in ≥2nd relapse in August 2017. Food and Drug Administration (FDA) approved Novartis' tisagenlecleucel (CTL-019, Kymriah), which is a synthetic bioimmune product of anti-CD19 chimeric antigen receptor (CAR) T cells, for the treatment of relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL).
Kymriah