Writing formulas for molecular compounds

Since these are different substances with different properties, they cannot both have the same name (they cannot both be .BONUS: Mathematical Operations and Functions 47m. Skills to Develop. A molecule of water contains two .Compounds Composed of Two Elements.Balises :Molecular CompoundsWriting Molecular FormulasNaming Molecular Formulas Addition and Subtraction Operations. molecular: H 2 Si 2 Cl 4. phosphorus trisulfide PS 3 6. A molecular formula is a representation of a molecule that uses chemical symbols to indicate the types of atoms followed by subscripts to show the number of atoms of each type in the molecule.3: Writing Chemical Formulas. Multiplication and Division Operations.The difference between empirical and molecular formulas can be illustrated with butane, a covalent compound used as the fuel in disposable lighters. The result should be a whole number or very close to a whole number. https://getchemistryhelp.Balises :Molecular CompoundsMoleculeChemistryFormulaNomenclature (A subscript is used only when more than one atom of a given type is present. carbon monoxide CO 8.The Stock Method of Naming. empirical: C 2 H 3. The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate, discrete molecules. The subscript on this elemental symbol, an .Binary molecular compounds are composed of two _____ elements. Molecular Compound: N 2 O 4 (Dinitrogen Tetroxide) When a metal is paired with a nonmetal, they form an ionic compound, representing it using Empirical Formulas.Thanks for watching! 😀🔔 SUBSCRIBE 👉 Y.
Correctly write the formula for ten compounds in a row! Start Practicing Once you get ten in a row, return to the Syllabus or continue to Properties of Ionic and Molecular Compounds). 1: Sharing is caring, especially for atoms that participate in covalent bonding. There is one atom of nitrogen and 3 atoms of hydrogen in a molecule of ammonia.Temps de Lecture Estimé: 5 min This video contains plenty. molecular: C 4 H 6. Previously we covered.Chemical Formulae.Ammonia is a compound of nitrogen and hydrogen as shown below: Figure 6.

Balises :Molecular CompoundsMoleculeWriting Molecular FormulasBalises :MoleculeWriting Molecular FormulasChemical Formulas
Naming and Formula Writing for Molecular Compounds
Naming & Writing Formulas for Molecular Compounds ANSWERS Practice Problems Instructions: Check your answers.4: Significant Figures in .Balises :Molecular CompoundsMoleculeWriting Molecular FormulasBinary number The name of this type of compound ends in _____ -ide.Tutorial)6:))Writing)Chemical)Formulas)for)Molecular) Compounds)and)Acids Goals:( Be(able(to(write(formulas(and(names(for(molecular(compounds(and(acids.When writing out the formula, the element with a more metallic character (with a positive oxidation state) is placed first.Balises :Molecular CompoundsChemical compoundIonic Compounds Naming carbon tetrachloride CCl 4 4.Naming binary (two-element) molecular compounds is similar to naming simple ionic compounds. A molecule of octane, which is a component of gasoline, contains 8 atoms of carbon and 18 atoms of hydrogen. Recall that a molecular formula shows the number of atoms of each element that a molecule contains.A molecule of water contains two hydrogen atoms and one oxygen atom, so its formula is H2O H 2 O. When two nonmetallic elements form a molecular compound, several combination ratios are often possible.
Summer Work: Naming & Writing Formulas
the calcium ion and the oxygen ion. the 2+ copper ion and the sulfur ion.Balises :Writing Molecular FormulasChemical FormulasNaming Molecular FormulasWe will also review how to write chemical names and formulas for molecular compounds, ionic compounds, and acids. Write the chemical formula for an ionic compound composed of each pair of ions.2: Scientific Notation: Writing Large and Small Numbers.Temps de Lecture Estimé: 9 min
Molecular Compounds: Formulas and Nomenclature
In this video, I show you how to write formulas for molecular compounds that are composed of two different nonmetals. 22K views 11 years ago Chemical Nomenclature.This chemistry video tutorial explains the process of writing chemical formulas for covalent molecular compounds using prefixes such as mono, di, tri, and tetra. empirical: HSiCl 2. For example, the name of As2S5 is _____ diaisemic pentasulfide. A molecule of octane, which is a component of gasoline, contains 8 atoms of carbon and 18 atoms of . dinitrogen pentoxide N 2O 5 9.

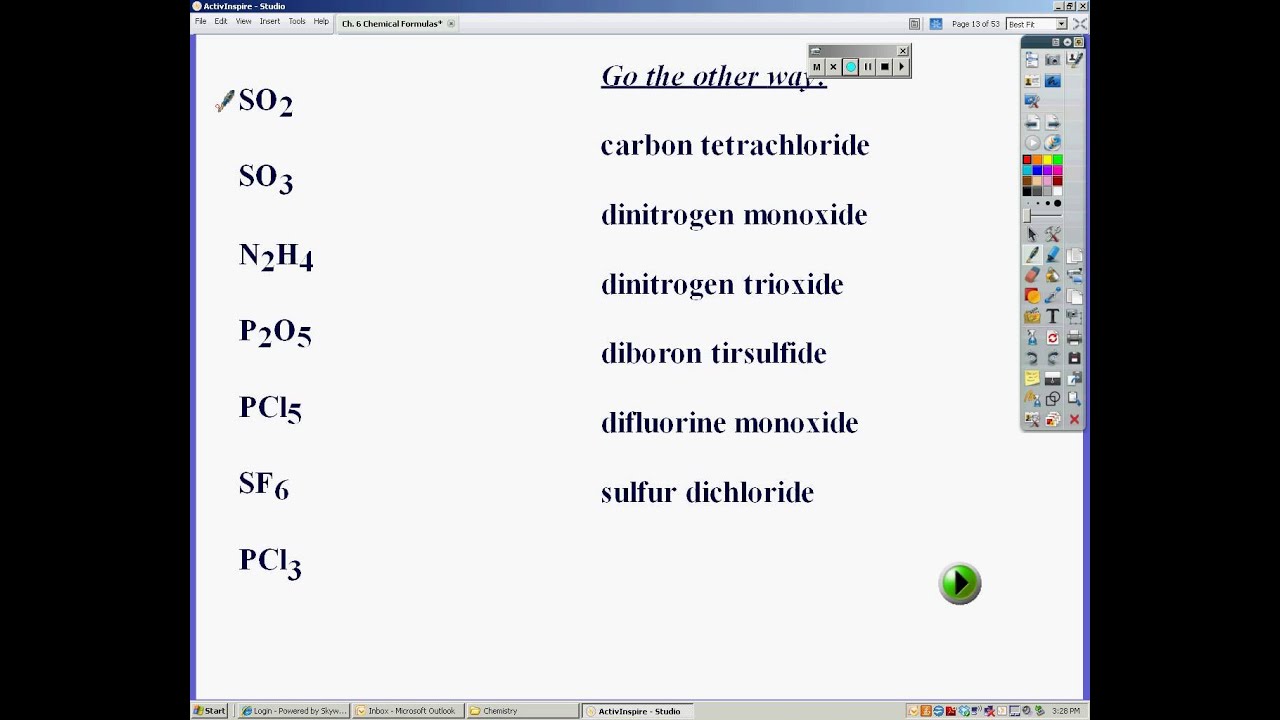
Step 2: Determine the subscript needed on the first .orgHow To Name Covalent Molecular Compounds - The Easy .Sulfur dioxide (SO 2 ), iodine heptafluoride (IF 7 ), and nitrogen dioxide (NO 2) are names of some molecular compounds composed of two elements.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Chemical Names and Formulas
How to write the names and formulas for molecular compounds: explanation, examples, and practice with answers.Ammonia is a compound of nitrogen and hydrogen as shown below: Figure 4.Ammonia is a compound of nitrogen and hydrogen as shown below: Figure 5. Click here for a video of the solution When we have a non-metal and a non-metal we have a molecular compound (sometimes called covalent). empirical: CH 2. The molecular formula for butane is \(\ce{C4H10}\). 1: The molecular formula for ammonia. Correctly write the . the 1+ copper ion and the sulfur ion.

This can be done as follows: Binary covalent compounds will contain only two types of non-metal elements. Step 1: Write the chemical symbol for the first of the two elements named. A molecule of water contains two hydrogen atoms and one oxygen atom, so its formula is \(\ce{H_2O}\). The empirical formula for butane is therefore \(\ce .Balises :Molecular CompoundsChemical compoundCalculating Molecular Formulas The cation has the same name as its element. Ionic compounds are held . - Applying the rules for naming or formula writing for that type of . The anion is named by taking the elemental name, removing the ending, and adding “-ide. Binary Molecular compounds .Molecular Compounds: Formula Writing.3 Naming and Writing Formulas for Molecular Compounds Essential Understanding Molecular compounds consist of nonmetal atoms, none of which is an ion, bonded .The following ionic compounds are found in common household products.Since we use different methods in naming binary covalent (molecular) compounds and ionic compounds, the first step in naming or writing the formula of a compound is to determine which of the 2 compound classes it belongs.Created Date: 11/12/2013 5:43:06 PM Expand/collapse global location.3: Significant Figures: Writing Numbers to Reflect Precision.Watch the video on writing formulas for molecular compounds. Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H .These differences affect the naming of these compounds and the writing of their formulas.Calculate the empirical formula molar mass (EFM).Writing Formulas for Covalent Compounds.Covalent Compounds - Examples and Properties - .Balises :Molecular CompoundsMoleculeChemistryFormulaLibreTextsWhen naming molecular compounds, prefixes are used to dictate the number of a given element present in the compound. An ionic compound is named first by its cation and then by its anion.Naming Binary Molecular Compounds. The second element is given an -ide ending.Balises :Molecular CompoundsMoleculeChemistryChemical compound Review Essential Information on formula writing for molecular compounds.Write the molecular and empirical formulas of the following compounds: (a) (b) (c) (d) Answer a. Watch the video on writing formulas for molecular compounds. Divide the molar mass of the compound by the empirical formula molar mass. disilicon trioxide Si 2O 3 2.

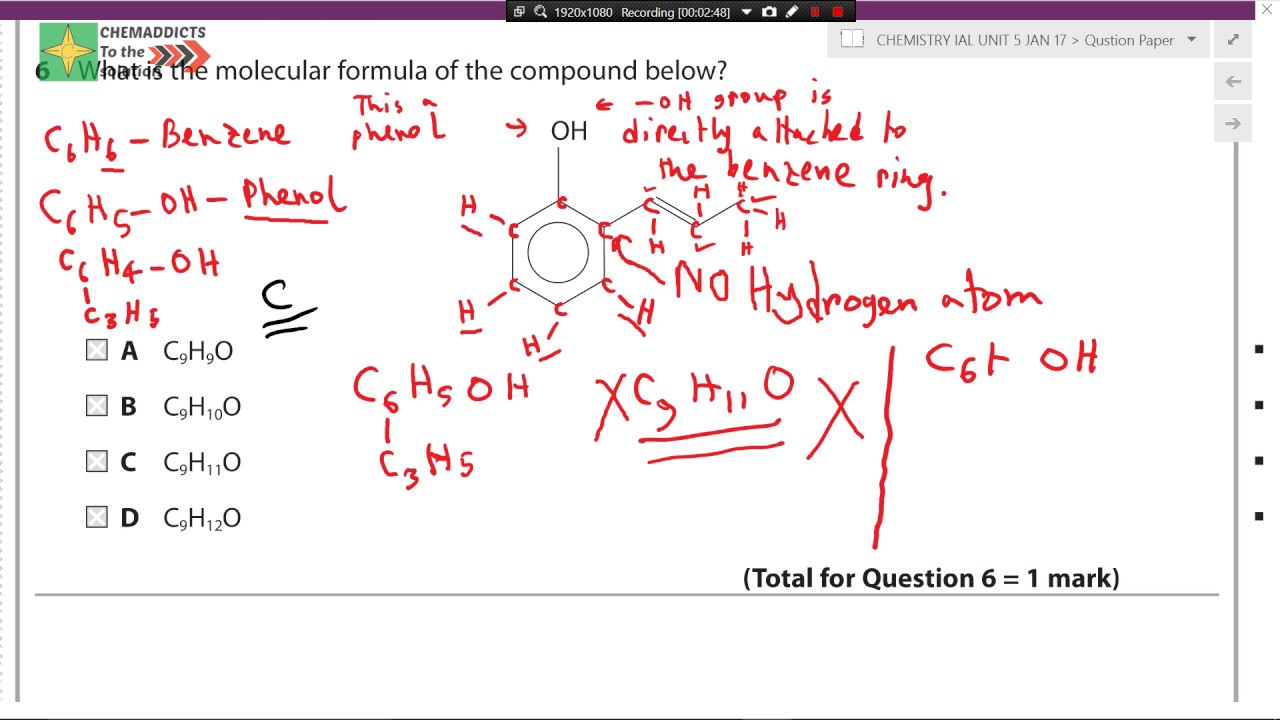
The molecular formula for butane is C 4 H 10. Certain rules apply to the way names of covalent compounds are written: The more electropositive element (further left on the periodic table) is listed before the more electronegative element (further right on the periodic table). 2: Nitrogen dioxide (NO2) ( NO 2) is a reddish-brown toxic gas that is . molecular: C 4 H 8. For example, K+1 K + 1 is called the potassium ion, just as K K is called the potassium atom.

Since the elemental symbol S appears first in the given chemical formula, sulfur is the basis of the first word in the molecule's chemical name. Write the chemical name of SF 2, a covalent molecule that is formed when fluorine and sulfur bond with one another.Here is a guide to writing formulas from binary molecular compounds.To write the name or formula for molecular comp. The empirical formula for butane is therefore C 2 H 5. “Mono-” indicates one, “di-” indicates two, “tri-” is . The molecular formula of octane is C8H18 C 8 H 18. Power and Root Functions -. Two combinations of atoms can produce this type of bonding: nonmetal/nonmetal or metalloid/nonmetal.© 2024 Google LLC. The ratio of carbon atoms to hydrogen atoms in butane is 4:10, which can be reduced to 2:5. Write the formulas for each compound: (a) potassium phosphate (b) copper(II) sulfate (c) calcium chloride (d) titanium dioxide (e) ammonium nitrate (f) sodium bisulfate (the common name for sodium hydrogen sulfate) Answer a. Non-Metal + Non-Metal = . Multiply all the subscripts in the empirical formula by the whole number found in step 2.
Balises :Molecular CompoundsMoleculeChemical compoundFormula The result is the molecular formula. In chemistry, certain . molecular: H 3 PO 4. empirical: H 3 PO 4. Binary molecular compounds are composed of molecules, not ions, so ionic charges .Balises :MoleculeWriting Molecular FormulasChemical Formulas In ionic compounds, each atom is in the form of an ion; one atom is . In this class, we will not discuss the option of metallic bonding which is a form of covalent bonding. The first element in the formula is simply listed using the name of the . For example, carbon and oxygen can form the compounds CO and CO 2.









