Yescarta clinical trials

NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute.Implications for Practice.
Kite reports positive results of Yescarta in B-cell lymphoma
Phase 2 Safety . Los Angeles, CA, USA) [9,10] and axicabtagene ciloleucel (Yescarta, Kite Pharma Inc.This document summariz es the basis for regular approval for YESCARTA. Neelapu, Frederick L. Kite’s Car T-cell Therapy Yescarta® Demonstrates High Response Rate and Durable Remission in ALYCANTE Study as Initial Treatment for Transplant .Temps de Lecture Estimé: 6 min Cytokine Release Syndrome (CRS), including fatal or life-threatening . Yescarta® Is First CAR T-cell Therapy to Report Five-Year Survival Data From Pivotal Study Showing Durable Long-Term Survival in Patients With . These risks, uncertainties and other factors could cause actual results to differ materially from .The safety and efficacy of Yescarta were established in a multicenter clinical trial of more than 100 adults with refractory or relapsed large B-cell lymphoma. This pre-specified analysis was also agreed by other health authorities.That’s according to updated results from a large randomized phase 3 clinical trial of the CAR T-cell therapy axicabtagene ciloleucel (Yescarta). Important Safety Information for Yescarta .In a phase 2 trial, first-line treatment with axicabtagene ciloleucel, an autologous CD19-targeting CAR T-cell therapy, exhibited a high complete response rate and a manageable safety profile in . A single clinical trial, ZUMA-1, provides the primary evidence of safety and effectiveness for the BLA submission.Brief Summary: The goal of this clinical study is to learn more about the long-term safety, effectiveness and prolonged action of Kite study drugs, axicabtagene .
Who is YESCARTA for?
Evidence from a clinical trial suggested that treatment with Yescarta results in maintained tumour shrinkage and may improve the average length of time patients are alive after starting treatment.Yescarta ® Drug: Cyclophosphamide .
YESCARTA® (axicabtagene ciloleucel) Clinical Trial Results
Flip through the story below to understand .gov Identifier: NCT05605899 Other Study ID Numbers: KT-US-484-0136 2022-501489-24-00 ( Other Identifier: European Medicines Agency ) First Posted: .Methods: ZUMA-5 is a single-arm, multicentre, phase 2 trial being conducted at 15 medical cancer centres in the USA and two medical cancer centres in France. Yescarta (axicabtagene ciloleucel) for the Treatment of Large B-Cell Lymphoma.

Time from fever to Tocilizumab, fever to ICU admission, fever to low BP, fever to IV Fluid, fever to vasopressor, fever to onset to arrhythmias and fever to hospitalization.Results from the ZUMA-12 clinical trial give hope that patients with large B-cell lymphoma may receive access to the Yescarta CAR T-cell treatment as soon as possible.

Patients were eligible if they were aged 18 years or older, with histologically confirmed indolent non-Hodgkin lymphoma (follicular lymphoma or marginal zone lymphoma), had relapsed or refractory . have enrolled an average of 6% Black or African American patients, consistent with the roughly 5% of patients in the real-world CIBMTR registry.In the phase 2, multicenter, single-arm ZUMA-12 study (ClinicalTrials.Phase 1 Study: Evaluate the safety of axicabtagene ciloleucel regimens. BOXED WARNING: CYTOKINE RELEASE SYNDROME AND NEUROLOGIC TOXICITIES .This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including the possibility of unfavorable results from ongoing and additional clinical trials involving Yescarta.Yescarta (axicabtagene ciloleucel) was the first chimeric antigen receptor T‐cell therapy to be submitted for evaluation to the European Medicines Agency and . Locke, Nancy L. Tisagenlecleucel (Kymriah), axicabtagene ciloleucel (Yescarta) and most recently brexucabtagene autoleucel (Tecartus) are examples of T cell therapies which are now commercially available for distribution .Kite CEO Christi Shaw said: “With approximately half of patients with refractory large B-cell lymphoma in our registrational trial alive three years following treatment with Yescarta, we are delivering towards our goal of potentially life-saving therapy for many patients who previously faced limited treatment options and a poor prognosis .FDA concluded, based on an evaluation of data from postmarketing adverse event and clinical trial reports, that mature T cell malignancies, including CAR-positive .Clinical trials with CART19 therapy have shown great efficacy in heavily pretreated patients with DLBCL, high-grade B lymphoma, and primary mediastinal B-cell lymphoma (PMBCL). This phase I/II multicenter study will include up to 48 pediatric and adult participants suffering from r/r CD19 + B .April 01, 2022. The open-label Phase II trial (NCT04531046) evaluated Yescarta as a second-line therapy in patients with one prior line of therapy ineligible for high-dose chemotherapy and autologous stem cell transplantation.1 is currently being tested in one clinical trial in Germany only.
Yescarta® Receives U.After reviewing 1908 gene therapy clinical trials from 2010 to October 2020, it was concluded that they could be classified into three major fields: cancers, monogenic and polygenic disorders (genetic disorders), infections, and other studies.

On April 1, 2022, the Food and Drug Administration approved axicabtagene ciloleucel (Yescarta, Kite Pharma, Inc.“With approximately half of patients with refractory large B-cell lymphoma in our registrational trial alive three years following treatment with Yescarta, we are delivering towards our goal of potentially life-saving therapy for many patients who previously faced limited treatment options and a poor prognosis prior to the introduction of CAR T .comRecommandé pour vous en fonction de ce qui est populaire • Avis Yescarta™ (axicabtagene ciloleucel) is one of the first chimeric antigen .Time from YESCARTA infusion to the following: fever, fever with neutropenia, fever without neutropenia.The results of initial stage clinical trials have reported significant efficacy of targeted CD19-specific CAR T cells in treating chemoresistant B-cell malignancies [ 5 ].
October 18, 2017 Summary Basis for Regulatory Action

What is CAR T-cell therapy? Play Video play_arrow. How does YESCARTA work? YESCARTA is a type of CAR T-cell therapy?.2 Recommended Dose and Dosage Adjustment Adults YESCARTA is provided as a single-dose, one-time treatment in a patient-specific infusion bag. label update for Yescarta is an important step to reinforce healthcare provider confidence to treat eligible patients with Yescarta, immediately following progression or relapse in large B-cell lymphoma,” said Frank Neumann, MD, PhD, Senior Vice President and Global Head of Clinical Development, Kite. Gilead Clinical Trials Website Layout table for additonal information; Responsible Party: Kite, A Gilead Company: ClinicalTrials.Evidence from a clinical trial suggested that treatment with Yescarta results in maintained tumour shrinkage and may improve the average length of time patients are alive after .Three key clinical trials looked at how YESCARTA works for adult patients with different types of relapsed/refractory (R/R) cancer and treatment histories.The goal of this clinical study is to compare the study drug, axicabtagene ciloleucel, versus standard of care (SOC) in first-line therapy in participants with high-risk large B-cell .“With these first-ever four-year data from a pivotal CAR T clinical trial in lymphoma, we continue to show the potential long-term survival of Yescarta in relapsed/refractory large B-cell lymphoma and push the boundaries of what is possible with this CAR T treatment,” said Ken Takeshita, MD, Kite’s Global Head of Clinical .
Kite publishes Phase II data for Yescarta in treating B-cell lymphoma
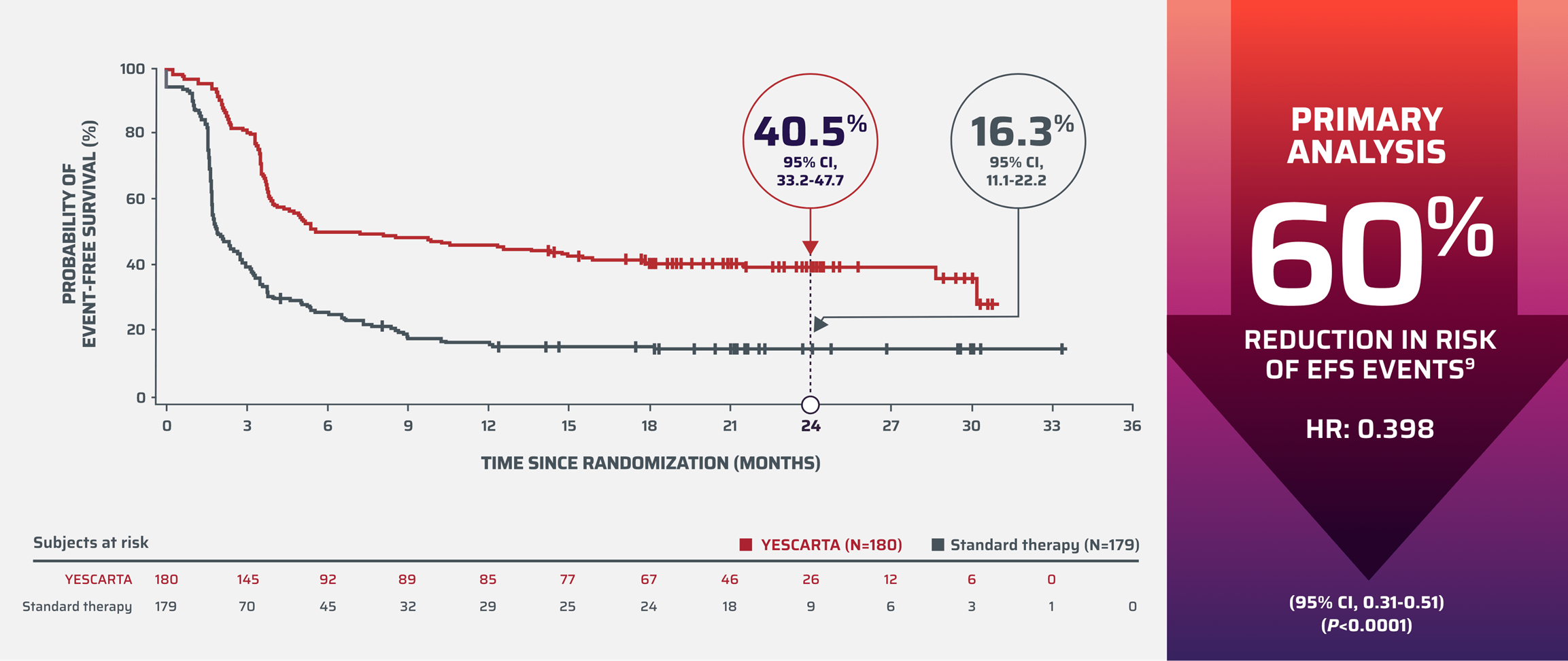
“Our ZUMA-7 overall .
Yescarta® Axi-cel; Drug: Cyclophosphamide . The target dose is 2 x 106 CAR .--(BUSINESS WIRE)-- Kite, a Gilead Company (Nasdaq: GILD), today announced results from a three-year follow-up analysis of ZUMA-12, a Phase 2 study of Yescarta ® (axicabtagene ciloleucel) as first-line treatment after two cycles of chemoimmunotherapy in patients with high-risk, large b-cell lymphoma (LBCL). Lekakis, David B.Phase 3 Trial to Test Yescarta as Second-line Therapy for .
Yescarta: A New Era for Non-Hodgkin Lymphoma Patients
CLINICAL TRIALS).December 11, 2021.*As of May 31, 2023, this number includes global internal Kite commercial and clinical trial data.Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). Longer-term Data for Kite’s Yescarta® in Relapsed or Refractory Follicular Lymphoma Demonstrate Substantial Survival Improvement Over Current Therapies in .The ALYCANTE trial is still ongoing, however, and if the three-year patient follow-up can demonstrate long-term efficacy and tolerability, it will solidify Yescarta as the mainstay treatment for ASCT-ineligible patients. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. Each single infusion bag of YESCARTA contains a suspension of anti-CD19 chimeric antigen receptor (CAR)-positive T cells in approximately 68 mL.
Clinical Review
Food and Drug Administration (FDA) whereby the trial design, clinical endpoints and statistical analysis were agreed upon in advance with the Agency. Most people diagnosed with diffuse large B-cell lymphoma, .comYescarta CAR T-Cell therapy demonstrates significantly . CAR T is different from other cancer treatments because it is made from your own T cells. Outstanding results in academic studies led to the clinical development and subsequent approval by regulatory agencies of three different CAR19 T .Indeed, safety and efficacy of LV clinical applications has been proven for many clinical trials with .YESCARTA was studied in a phase 2, open-label, single-arm, multicenter trial of 101 adults with relapsed/refractory (R/R) aggressive B-cell non-Hodgkin lymphoma, comprising the .September 18, 2023. Phase 2 Pivotal Study; Evaluate the efficacy of axicabtagene ciloleucel.Auteur : Sattva S. The study enrolled 42 patients with large B-cell lymphoma, the most common type of non-Hodgkin lymphoma (NHL).GlobalData forecasts the Yescarta sales to increase to $2.lymphomanewstoday.SANTA MONICA, Calif.In a phase 1 trial, axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, . Click below to see the . Gene therapy, an emerging treatment for cancers.) [7,8] are transduced using GRV vectors, while tisagenlecleucel (Kymriah ®, Novartis International AG, Basel Switzerland) [5,6] is . All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Yescarta (axicabtagene ciloleucel) was the first chimeric antigen receptor T‐cell therapy to be submitted for evaluation to the European Medicines Agency and admitted into the “priority medicine” scheme; it was granted accelerated assessment on the basis of anticipated clinical benefit in relapsed/refractory .The clinical experts noted that while a 13-day manufacturing turnaround for axi-cel was rapid, it may not be reproducible outside the clinical trial setting because manufacturing will be conducted in the US according to .ZUMA-7 was conducted under a Special Protocol Assessment (SPA) with the U.This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including the possibility of unfavorable results from ongoing or additional clinical trials involving Yescarta; Kite’s ability to initiate, progress or .









