Ruxolitinib patient assistance

Insurance benefit verification.
Connecting eligible patients to support and resources
This medication is used to treat certain bone marrow disorders . Myelofibrosis is a life-threatening bone marrow problem which is manifested by the following symptoms: enlarged spleen (splenomegaly), severe .IncyteCARES may help eligible patients with: Reimbursement Support.Studies in cell lines, primary colony assays, and in vivo models suggest ruxolitinib targets signaling downstream of oncogenic CSF3R.
Have a valid prescription for .2%) in TRuE-V2 who applied ruxolitinib cream for 52 weeks and in 26 of 82 patients (32%) and 18 of .Vitiligo repigmentation during treatment with ruxolitinib.
Prescription Assistance Program for Jakafi® (ruxolitinib)
Jakafi is used to treat adults and children 12 years of age and older with acute graft-versus-host disease (GVHD) who have taken corticosteroids . IncyteCARES for Jakafi Home. A resource to help physicians, advocates, and patients access .
RxAssist
A retrospective analysis of 13 patients diagnosed as HLH who received treatment with emapalumab combined with ruxolitinib at our center was conducted .The patient experienced a rapid improvement in sense of well-being and pruritus, and within 12 weeks, her spleen volume was reduced by 47%.
OPZELURA® (ruxolitinib)
You can also email for questions or to . Ces enzymes sont impliquées dans la signalisation de différents facteurs de croissance et cytokines qui sont importants pour l'hématopoïèse et la fonction immunitaire. The Yours Truly Support Program is all about making . (A) Study flowchart.Ruxolitinib (phosphate) 10 mg comprimé.Low blood counts: Jakafi ® (ruxolitinib) may cause low platelet, red blood cell, and white blood cell counts.Ruxolitinib is a janus-activated kinase inhibitor (JAK) that selectively inhibits the JAK1 and JAK2 protein kinases. Screening skin examination reveals near-complete depigmentation of the patient’s face at baseline.For Eligible Patients Who Are Uninsured or Underinsured for Jakafi® (ruxolitinib) IncyteCARES for Jakafi Patient Assistance Program.Les patients se soumettent à des prises de sang régulières, qui permettent de vérifier la numération globulaire et de surveiller les fonctions hépatique et rénale.Le ruxolitinib est un inhibiteur sélectif des Janus kinases (JAK) JAK1 et JAK2 (valeurs CI 50 de respectivement 3,3 nm et 2,8 nm pour les enzymes JAK1 et JAK2).2% of patients achieved hematocrit control with 92.Of the 120 patients with anemia, mean age was 64.Patient disposition, duration of treatment, and ruxolitinib exposure. When prompted, provide the conference identification number, 383585.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Patient Assistance Program
1% to 10%, and patients requiring ≥3 phlebotomies decreased from 31. Ruxolitinib Tablet - Uses, Side Effects, and More. [1] It is an oral medication approved by the FDA to treat high-risk myelofibrosis, .Yours Truly Patient Support | Atopic Dermatitis | OPZELURA® (ruxolitinib) Patient support that's truly there for you.
IncyteCARES for Jakafi Patient Support Program
No new or unexpected safety signals were identified.

To access the conference call, please dial 877-407-8037 for domestic callers or 201-689-8037 for international callers.

Treatment-related .
Yours Truly Patient Support
Patient Assistance . Contact phone number (s) .Patient Assistance Program | IncyteCARES for Jakafi® (ruxolitinib) Home. INDICATIONS ET MODALITÉS D'ADMINISTRATION. IncyteCARES for OPZELURA Patient Assistance Program For uninsured or underinsured Medicare Part D patients only Complete pages 1, 2, and 3.
Patient Assistance Program
Pharmacokinetic data of three clinical trials, with different dosing strategies, were used to make a popPK model. 2019 Mar;156(4):1206-1210. No substitutions are permitted.Patient assistance may be available to qualifying individuals without prescription drug coverage.Learn about treatment with OPZELURA® across all uses on the official patient website. Ruxolitinib is used to treat intermediate or high-risk myelofibrosis, including primary myelofibrosis, post-polycythemia vera myelofibrosis and post-essential thrombocythemia myelofibrosis. Your care team can help you find these resources if they are available. 2 of 4 PRESCRIPTION AND ENROLLMENT FORM OR .Purpose: Colony-stimulating factor-3 receptor (CSF3R)-T618I is a recurrent activating mutation in chronic neutrophilic leukemia (CNL) and to a lesser extent in atypical chronic myeloid leukemia (aCML) resulting in constitutive JAK-STAT signaling.Learn about Jakafi® (ruxolitinib) – Used to treat adults with polycythemia vera who have taken hydroxyurea and it did not work well enough or they could not tolerate it, and adults with certain types of myelofibrosis. See Important Safety Information and Full Prescribing Information, including Boxed .In anemic subgroups, momelotinib was associated with higher rates of transfusion independence and reduced/stable transfusion intensity vs.Medication Name: OPZELURA ( ruxolitinib) cream , 1.If you have prescription drug coverage, we can help guide you through the reimbursement process for certain Novartis medications listed below.Most patients (59.Patient agrees that they will not in any way report or count the value of the Jakafi® (ruxolitinib) provided under this program as true out-of-pocket (TrOOP) spending under a Medicare Part D prescription drug benefit.9% from baseline to end . Access and Support. Ruxolitinib belongs to a group of targeted therapy drugs known as protein kinase inhibitors. Guidance with appealing .
Patient Assistance Program
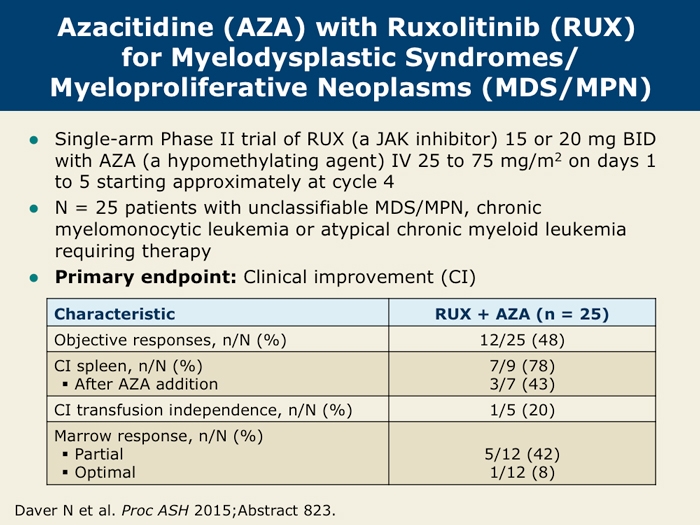
ORR by protocol-defined criteria for the first 25 patients was 32% (8/25 with . Co-pay cards, which reduce the patient co-pay responsibility for eligible, commercially (non-government sponsored) insured patients, are also available. We’re Here to Support Your Eligible .To enroll in the copay assistance program, call 1-855-452-5234, Monday through Friday between 8:00 AM and 8:00 PM ET.We sought to evaluate safety and efficacy of the JAK1/2 inhibitor ruxolitinib in patients with CNL and .In comparison, no patients remained on BAT; 8 of 9 (89%) BAT-randomized patients crossed over to receive ruxolitinib and 1 discontinued for splenectomy. REACH2 (NCT02913261; N=309) is a randomized, phase 3 trial investigating the efficacy and safety of the Janus kinase (JAK) 1/JAK2 inhibitor ruxolitinib (RUX) vs best available therapy (BAT) in pts with steroid .If you experience any of the following side effects, call your doctor or get emergency medical treatment immediately: crushing chest pain or chest heaviness; shortness of . Dernière modification : 13/05/2022 - Révision : 12/05/2022. Overall, momelotinib provides spleen, symptom, and anemia benefits to JAK inhibitor-naive patients with myelofibrosis regardless of .2%) in TRuE-V1 and 87 of 177 patients (49. Copay/coinsurance assistance* Free medication program; Temporary access for insurance coverage delays † Information about independent . Management: After 12 weeks of ruxolitinib therapy, her hemoglobin levels decreased from a baseline value of 12.Ruxolitinib (Jakavi ®) is used to treat some myeloproliferative neoplasms (MPNs).2% were female, and 60.Physicians abstracted patient demographic and clinical characteristics at diagnosis and during ruxolitinib and fedratinib therapy (Supplemental Figure A1), including bone marrow fibrosis grade, biomarkers, risk assessment score, Eastern Cooperative Oncology Group performance status (ECOG PS), dose, dose modifications (reductions, . Screening skin examination reveals near-complete depigmentation of the patient's face at baseline. Helping Eligible Patients With.0% were White; 3. The 7 adverse events, regardless of treatment, that led to discontinuation were severe blood creatine phosphokinase increase, severe gastrointestinal disorder, Kaposi sarcoma, moderate left-foot pain, severe multiorgan failure, moderate renal . Generic Name: ruxolitinib. In agreement with the hematologic laboratory abnormalities over time in the ruxolitinib-randomized group, mean hemoglobin levels decreased during the first 12 weeks of treatment with . Healthcare Professionals.Step 1: Complete the Patient Information (or have your patient complete it) including: Patient shipping address for medication delivery.OPZELURA is indicated for the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis in non-immunocompromised adult and pediatric patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Of the eight patients who crossed over to receive ruxolitinib, six discontinued due to disease progression ( n = 2), adverse events ( n = 2), study medication non-adherence ( n = 1) or .1,2 Here we report a case of .Patient assistance options.The IncyteCARES for Jakafi Patient Assistance Program (PAP) helps eligible patients who do not have prescription drug insurance or have trouble affording . (Novartis) has received approval from Health Canada for Pr JAKAVI® (ruxolitinib tablets), a drug for the treatment of disease-related splenomegaly (enlarged spleen) and/or its associated symptoms in adult patients with myelofibrosis (MF), a blood cancer.3 g/dl, and her platelet levels decreased to 137 × 10 9 /I.Find out if you qualify for the IncyteCARES for Jakafi Savings Program.
Financial Assistance Support for Patients
Limitations of Use: .
The first evidence of skin repigmentation appeared after 12 weeks of therapy, which continued until week 20, when ruxolitinib was discontinued. Ruxolitinib is used to treat myelofibrosis and polycythaemia vera. 1 Most programs offer valuable . These are the most common side effects, but you might have others.1% at baseline to 85.1% of patients had no phlebotomies during the study. Follow-up visit 12 weeks after .Fig 1 Vitiligo repigmentation during treatment with ruxolitinib.
Manquant :
patient assistancePatient Assistance Program | IncyteCARES for OPZELURA® (ruxolitinib) Helping Eligible Patients With.
Results: Of 176 patients who received ruxolitinib as first MF treatment, 120 had anemia at diagnosis; baseline demographics and patient characteristics are shown in Table 1. If you are outside the Memphis area, dial toll-free 1-866-2STJUDE (1-866-278-5833 .Efficacy of Ruxolitinib Therapy in a Patient With Severe Enterocolitis Associated With a STAT3 Gain-of-Function Mutation. It targets genes called JAK1 .A T-VASI50 response occurred in 92 of 173 patients (53.Drugs & Medications. Information about prior authorization requirements. If you develop bleeding, stop taking Jakafi and call your healthcare .

4% achieved hematologic remission at month 24.Jakafi (Ruxolitinib) - $60.
Manquant :
patient assistanceFinancial Assistance and Support for Jakafi® (ruxolitinib)
3% patients having hematocrit <45% and 35. Efficacy of Ruxolitinib Therapy in a Patient With Severe Enterocolitis Associated With a STAT3 Gain-of-Function Mutation Gastroenterology.
Pharmacokinetics and Pharmacodynamics of Ruxolitinib: A Review
Find how the IncyteCARES Patient Assistance Program for OPZELURA may help eligible uninsured or under-insured patients with Medicare Part D coverage.
JAKAVI® (ruxolitinib) Fact Sheet
For Patients Whose . Follow up visit 12 weeks after stopping the treatment .